
- Other Species
Destinations
- Sightseeing
- Testimonial
- Bow Hunting

- Testimonials
- Monday : 09:00 - 17:00
- Thusday : 09:00 - 17:00
- Wednesday : 09:00 - 17:00
- Thursday : 09:00 - 17:00
- Friday : 09:00 - 17:00
- Saturday : Closed
- Sunday : Closed
Address : M. Kasapoglu cd. 24/5, 07160 Antalya-TURKEY
Phone : +90 242 322 00 27
Email : [email protected]

Data, analysis, convening and action.
The world’s largest and most diverse environmental network.

- IUCN WORLD CONSERVATION CONGRESS
- REGIONAL CONSERVATION FORA
- CONTRIBUTIONS FOR NATURE
- IUCN ENGAGE (LOGIN REQUIRED)
IUCN tools, publications and other resources.
- News & Events
- Eastern and Southern Africa
- Eastern Europe and Central Asia
- Mediterranean
- Mexico, Central America and the Caribbean
- North America
- South America
- West and Central Africa
- IUCN Academy
- IUCN Contributions for Nature
- IUCN Library
- IUCN Red List of Threatened Species TM
- IUCN Green List of Protected and Conserved Areas
- IUCN World Heritage Outlook
- IUCN Leaders Forum
- Protected Planet
- Union Portal (login required)
- IUCN Engage (login required)
- Commission portal (login required)
Get Involved

Wild sheep and goats and their relatives : status survey and conservation action plan for Caprinae

Wild caprinae, including sheep and goats, are an extremely valuable group of mammals. While most live in mountains, some inhabit desert grasslands, tropical forests or even arctic tundra. They range in size from the 30kg goral to the 350kg musk ox and display a variety of horn shapes and sizes as well as coat and body coloration. They are highly prized by hunters on account of their horns and their coats. Today, despite their important domestic relations, many wild caprinae are in danger of being lost forever: over 70 of caprinae taxa are threatened and over 30 endangered or critical. The main threats to them are over-harvesting, habitat loss and resource competition from livestock. Some face an additional threat from trophy hunters. Despite this, however, conservation legislation is either absent or, more often, poorly enforced. This action plan explores the value of caprinae to biodiversity, the threats facing the members of the species, and makes recommendations to reverse current trends. It also emphasises the importance to carpinae conservation and survival of close collaboration among all parties involved in wildlife conservation including local peoples and hunting organisations, governments, scientists and academic institutions.

Distributed in the Sundaland region (Thai-Malay Peninsula, Sumatra, Borneo, Java and adjacent…

This European Red List provides an updated summary of the conservation status of the European…

The IUCN Red List of Ecosystems is the global standard for ecosystem risk assessment and a …
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 15 August 2022
The abundance and persistence of Caprinae populations
- Grant M. Harris 1 ,
- Matthew J. Butler 1 ,
- David R. Stewart 1 &
- James W. Cain III 2
Scientific Reports volume 12 , Article number: 13807 ( 2022 ) Cite this article
1021 Accesses
4 Altmetric
Metrics details
- Conservation biology
Population dynamics
Stable or growing populations may go extinct when their sizes cannot withstand large swings in temporal variation and stochastic forces. Hence, the minimum abundance threshold defining when populations can persist without human intervention forms a key conservation parameter. We identify this threshold for many populations of Caprinae , typically threatened species lacking demographic data. Doing so helps triage conservation and management actions for threatened or harvested populations. Methodologically, we used population projection matrices and simulations, with starting abundance, recruitment, and adult female survival predicting future abundance, growth rate (λ), and population trend. We incorporated mean demographic rates representative of Caprinae populations and corresponding variances from desert bighorn sheep ( Ovis canadensis nelsoni ), as a proxy for Caprinae sharing similar life histories. We found a population’s minimum abundance resulting in ≤ 0.01 chance of quasi-extinction ( QE ; population ≤ 5 adult females) in 10 years and ≤ 0.10 QE in 30 years as 50 adult females, or 70 were translocation (removals) pursued. Discovering the threshold required 3 demographic parameters. We show, however, that monitoring populations’ relationships to this threshold requires only abundance and recruitment data. This applied approach avoids the logistical and cost hurdles in measuring female survival, making assays of population persistence more practical.
Similar content being viewed by others

Elk population dynamics when carrying capacities vary within and among herds

Weak evidence of density dependent population regulation when using the ability of two simple density dependent models to predict population size
Rapid population growth and high management costs have created a narrow window for control of introduced hippos in Colombia
Introduction.
Worldwide, Caprinae populations receive much attention given their coveted trophy hunts and iconic symbolism for wild and remote places, yet many Caprinae populations are at risk of extinction 1 . For biologists to protect and restore Caprinae populations threatened with extinction, and manage populations not threatened, they often apply logistically difficult and sometimes controversial techniques such as harvest, predator control, and translocation. Appropriate execution of these techniques requires foundational information describing a Caprinae population’s chance of extinction, demographic weaknesses, and minimum abundance requirements.
Populations with demographic rates producing stable to growing populations may decline to extinction when their sizes cannot withstand large demographic swings from temporal variation and stochastic forces 2 . Therefore, understanding the minimum population size at which a population can persist without (or minimal) human intervention forms a key conservation parameter. For Caprinae , the minimum abundance target for adult females enables biologists to triage conservation actions across populations 3 . Conservation resources can focus on rebuilding threatened populations below the threshold, and non-threatened populations may be hunted or serve as sources for translocation. Relatedly, this metric assists threat evaluations, as populations above the minimum threshold are more resilient to local extinction events 4 .
We identify this minimum abundance threshold describing population persistence for Caprinae . Methodologically, we perform a population viability assessment (PVA; e.g. 5 , 6 ), by developing a population projection matrix model to simulate population responses (population size and growth (λ) through time). We use matrix population models, as they form a conventional tool for predicting the growth of animal populations based on demographic parameters such as births, deaths, and age-structure 7 , 8 . The matrix approach is also commonly applied for identifying and assessing conservation strategies for endangered species 9 , 10 .
Although the 32 extant, autochthonous species of Caprinae have diverse behaviors, body sizes and inhabit different ecosystems, all females birth one offspring per year, by their second to third year (until death; twinning common in some species) 1 . We built our population viability model for Caprinae off these defining characteristics.
The urgency of conservation actions for most Caprinae populations far outpaces the rate of data collection 1 . Hence, the majority of Caprinae lack demographic information required to estimate population viability (e.g. mean and variability estimates of adult female survivorship and recruitment). We overcome the data deficiencies by simulating results using multiple combinations of starting abundances (varying from 10 to 250 adult females), mean recruitment (spanning 0–0.90) and adult female survivorship (0.50–0.98). Our simulations incorporated biological (temporal and environmental) variation and stochasticity, thereby quantifying uncertainties in future predictions. Estimating parameter variability relies on long-term datasets 11 , also nonexistent for most Caprinae 1 . Therefore, we quantified variation in recruitment and survivorship using data from prior field surveys and peer-reviewed literature on desert bighorn sheep ( Ovis Canadensis nelsoni ), as a proxy for other Caprinae .
The parameter values and combinations we employ correspond to empirical values reported from many different Caprinae populations. Indeed, estimating the minimum viable population (MVP) by evaluating a spectrum of biologically plausible values covers the differences in Caprinae social systems and environments influencing a given populations survival and recruitment values. We never assume that values for all Caprinae are similar, nor do we consider data from desert bighorn sheep representative for all Caprinae . We included model code and all simulation output, so any user can select demographic values of choice and examine the resulting changes in population persistence, or tailor an investigation to a specific population of interest.
Despite the copious literature defining minimum population sizes and building demographic simulations for a variety of species, prior studies rarely consider the practicality that managers require to inform on-the-ground work 12 . Such answers hinge on using data that managers can logistically acquire, affordably. Herein we implement this applied and practical approach.
We quantify and report the relationships between recruitment and adult female survival with population growth (λ), and therefore, the combinations of recruitment and survivorship required to attain a given λ, as recruitment and survival have most influence on Caprinae abundance 13 , 14 . Recruitment represents the number of offspring produced by reproductive females, each year, entering the yearling age class. Acquiring data describing recruitment is inexpensive and logistically simple. In contrast, survival data are usually derived from catching, marking (i.e., telemetry collars) and following adult females. The work is logistically challenging and expensive, making data describing survivorship scarce. It is also unlikely that biologists will acquire survivorship data for many Caprinae anytime soon. Therefore, while our simulations incorporate survivorship data, monitoring for the threshold abundance does not. Instead, we simplify results so biologists interested in determining when a population is secure from localized extinction can rely solely on accurate measures of abundance, abundance trend and recruitment.
Our results identify which demographic parameter generates the greatest impacts for increasing growth rate (λ) to recover an ailing population. We also show effects of translocation (adult female removals) on population abundance and trend of the source population, to identify populations sizes at which translocation is not detrimental. Lastly, we assess if survival or recruitment are useful for evaluating the efficacy of management actions, as the variability inherent to these parameters challenges such assessments.
For Caprinae managers, our results likely represent the sole information describing population projections and minimum abundance thresholds for the endangered Caprinae populations under their charge. For other wildlife and conservation practitioners, we exemplify a procedure for modeling population trajectories and identifying demographic thresholds for species with limited data.
Demographic parameters
Wildlife agencies often produce lamb:adult female ratios (i.e. recruitment) from surveys. To quantify variability in recruitment, we analyzed recruitment data of desert bighorn sheep from three state agencies (USA): the Arizona Game and Fish Department (AZGFD), the California Department of Fish and Wildlife (CAFW), and the New Mexico Department of Game and Fish (NMDGF). The yearlings of Caprinae , of either sex, can be challenging to identify as their size and horn characteristics often mimic adult females. Agencies handle this situation differently. The CAFW and NMDGF report the number of yearling females identified but omit yearling males and unclassified yearlings. The AZGFD reports the number of male, female and unclassified yearlings. The NMDGF includes yearling females in the denominator of the lamb:ewe ratio but CAFW and AZGFD do not. Across the three states, the lamb:ewe ratios that omit yearlings from the denominator (L:E) and those that include yearlings in the denominator (L:EY) are generally similar (Table 1 ).
When yearlings counts are removed from the denominator of the L:E ratio, an unknown, incomplete and inconsistent fraction of yearlings are actually removed from each survey. For example, 46% of surveys for calculating L:E ratios from NMDGF (35 site/years) and 75% from CAFW (107 site/years) have ≤ 1 female yearling reported. In Arizona, 48% of surveys have ≤ 1 female yearling counted, 47% have ≤ 1 male yearling counted, 85% have ≤ 1 unclassified yearling counted, and 21% have ≤ 1 yearling counted in all three yearling categories (max of 3 total yearlings; 615 site/years). Across the 3 states, 38% of surveys recording ≥ 50 ewes do not report any female yearlings, and 70% report ≤ 5 female yearlings. The simplest (by avoiding misclassification issues) and most comprehensive (contains all yearling animals observed) correction is including yearlings in the recruitment ratio (lamb:(ewe + yearling); L:EY).
We estimated mean recruitment (L:EY) as 0.294 with a biological variance of 0.028 ( n = 757; CV = 0.57). The mean and variance using the traditional L:E ratio (i.e., assumed exclusion of all yearlings from the denominator) was 0.33 and 0.033, respectively. Our subsequent analyses always used the biological variance with yearlings included in the ratio denominator.
The mean recruitment we employ aligns with values reported from other populations of Caprinae, and the variability in recruitment matches the reduced versions of variability we tested. For instance, populations of Dall’s sheep ( O. dalli dalli ) have lamb:(ewe + yearling) ratios ~ 0.30 with CVs exceeding 0.4 15 . Mountain goats ( Oreamnos americanus ) have mean kid:(yearling + nanny) ratios of 0.33 and CV = 0.21 (calculated from Table 1 found in 16 ). Punjab urial ( Ovis vignei punjabiensis ) can have mean lamb:ewe ratios of 0.44 with CV = 0.18 17 , and Astor markhor ( Capra falconeri falconeri ) have mean kid:(yearling + nanny) ratios of 0.32 and CV = 0.30 (number of females ≥ 20; N = 8 18 ). The variability in our recruitment data is likely higher than reported elsewhere for many other Caprinae populations, because our method accounts for inter-annual variability in the calculation (not calculating the mean variance).
We estimated the mean survival for adult female desert bighorn sheep as 0.82, with biological variance = 0.0127 (CV = 0.14; 31 site/years). This mean and variance in survivorship also corresponds with other Caprinae populations, such as Soay sheep ( Ovis aries ; mean = 0.87; CV = 0.15 [winter period]), mouflon ( Ovis gmelini ; mean = 0.84; CV = 0.13), and Dall’s sheep (mean = 0.88; CV = 0.11) 13 , 19 , 20 , 21 .
Simulations and minimum abundance of adult females
We quantified the probabilities of λ < 1 and quasi-extinction at year 10 and 30 ( QE (10); QE (30)), with different combinations of mean adult female survival, mean recruitment, and starting abundance of adult females, with the variance values fixed (Fig. 1 ). To achieve a stable population (λ ~ 1.0), the lowest recruitment can become is 0.20, provided an adult female survival of 0.98 (deterministic λ = 1.01; Table 2 ; Fig. 1 ). Alternatively, recruitment could be as high as 0.45 with a corresponding survivorship of 0.75 (deterministic λ = 1.01; Table 2 ; Fig. 1 ).
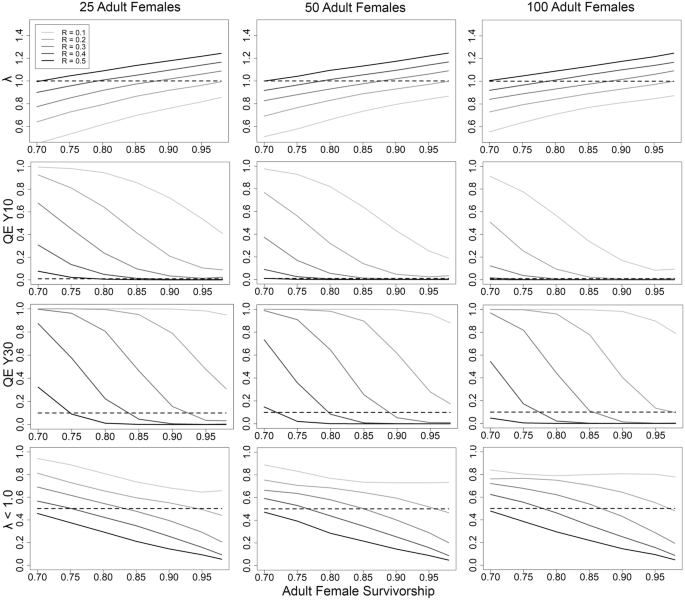
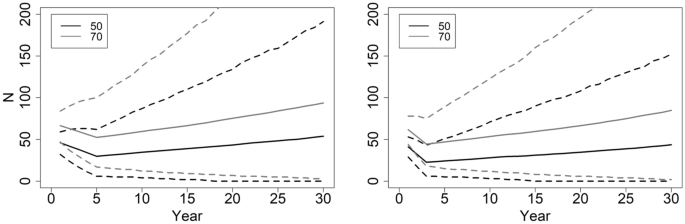
Relationships between recruitment (R), survival of adult females, starting adult female abundance, and population growth (λ; top row), the probability of quasi-extinction ( QE ; adult female abundance ≤ 5) at years 10 and 30 (middle rows) and the proportion of simulations resulting in mean λ < 1.0, based on 10,000 stochastic simulations of population trajectories. Dashed lines for QE (10) and QE (30) indicate the persistence threshold: 0.01 and 0.10 respectively.
The variability surrounding λ is high, with confidence levels often spanning ± 30% (Table 2 ). The likelihood of QE helps represent this variability. Acquiring QE (10) ≤ 0.01 with survivorship ≥ 0.85, requires a population of 25 adult females with recruitment ≥ 0.4, a population with 50 adult females with recruitment ≥ 0.3, and 100 adult females with recruitment approximately ≥ 0.2 (Fig. 1 ). A QE (30) ≤ 0.10 occurs with survivorship ≥ 0.80 and recruitment ≥ 0.4 with 50 adult females, and survivorship ≥ 0.85 and recruitment ≥ 0.3 with 100 adult females (Fig. 1 ).
We also examined variability by quantifying the proportion of scenario iterations resulting in λ < 1 (Fig. 1 , Table 2 ). Starting abundance has relatively small effects on this probability (Fig. 1 ). Attaining a ≤ 25% chance of population decline (stochastic λ) requires combinations of recruitment and survivorship such as survivorship ≥ 0.95 and recruitment 0.30, survivorship ≥ 0.90 and recruitment 0.40, or survivorship ≥ 0.85 and recruitment 0.50 (Fig. 1 ). Attaining a ≤ 10% chance of population decline requires survivorship ≥ 0.95 and recruitment ≥ 0.50 (Fig. 1 ).
We determined the minimum abundance of adult females required for ensuring population persistence, when the population has demographic rates consistent with a stable to slightly growing population (stochastic λ ~ 1; Supplementary Data S1 ). Results focused on QE , because starting abundance strongly affects this probability. With 25 adult females, populations have QE (10) ranging 0.03–0.09 and QE (30) 0.16–0.31 (Table 2 ). A population of 50 adult females must have intermediate levels of recruitment (0.25–0.40) and survivorship (0.80–0.95) for QE (10) ≤ 0.01 and QE (30) ≤ 0.10 (Table 2 ). With a starting abundance of 100 adult females, a population has QE (10) < 0.01 and QE (30) ≤ 0.10 for all simulations. For all starting abundances, the risk of QE increases with high recruitment and low survival (and vice-versa; Table 2 ). Based on these results, a population of 50 adult females forms a reasonable target to ensure population persistence.
QE increases when populations exhibit modest decline, as when survivorship is 0.85 and recruitment 0.30, generating λ ~ 0.97. With λ in slight decline, the QE (10) risk for a population of 50 adult females remains nearly identical to simulations with λ ~ 1.0, yet QE (30) rises ~ 5 times (Tables 2 , 3 ; Figs. 1 , 2 ). A population of 100 adult females with λ ~ 0.97 has QE (30) 10 times larger than when λ ~ 1.00 (Tables 2 , 3 ; Figs. 1 , 2 ). With λ ~ 0.97, populations having a starting abundance of 60 or 70 adult females decline to 50 by year 8 and 16 respectively. Therefore, a population of 50 adult females remains a minimum threshold target, provided that frequent and accurate monitoring of abundance catches any decline.
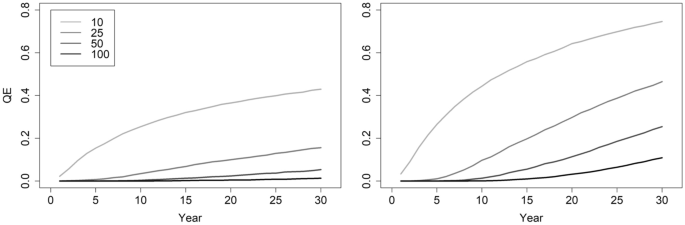
Plots portraying the probability of quasi-extinction ( QE ; mean abundance ≤ 5 adult females) of adult females based on 10,000 stochastic simulations of population trajectories. The left panel locks recruitment to 0.30 and survivorship at 0.90 (stochastic λ ~ 1.0) and the right panel has recruitment and survivorship at 0.30 and 0.85 respectively (stochastic λ ~ 0.97). Inset values indicate the starting abundances of adult females.
Biological variation influences the potential trajectories of population growth or decline. Higher variability means more alternative paths, so reductions in variability typically provide greater assurances in a population’s projection. We found that arbitrarily reducing biological variation by half for recruitment or adult female survival barely changed the resulting variability surrounding λ (Table 4 ). Nor does this reduction in variability substantially influence a population’s trajectory (Fig. 3 ). Keeping biological variance for one parameter and eliminating variance for the other, or halving biological variation for both parameters, reduces variability surrounding a stable λ to 20%, with little effect on population trajectories (Table 4 ; Fig. 3 ). Reducing variability by 90% or eliminating it for both parameters generated confidence intervals spanning ~ 10% around a stable λ (Table 4 ; Fig. 3 ). Without biological variance, a population of 50 adult females could decline to 40 or increase to 90 adult females by year 10 (Fig. 3 ). Increases in biological variation raise QE , but effects do not manifest at year 10 ( QE (10) always 0), and QE (30) changes are minimal (Table 4 ).
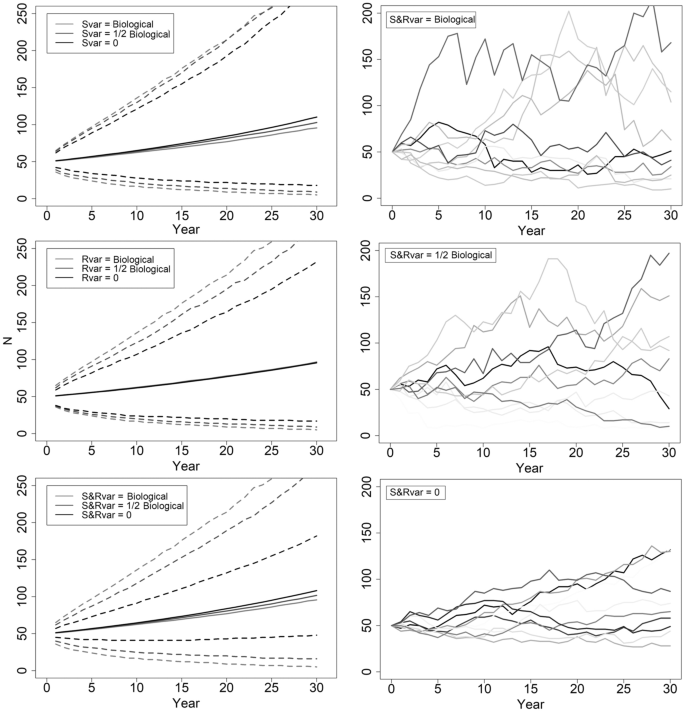
The effects of variability in adult female survivorship and recruitment parameters on the abundance estimates for adult females. Mean abundances are solid lines with dashed lines representing 90% confidence intervals. Upper left: Recruitment variance held at the biological value (0.028), and survivorship variance takes the biological value (0.0127), halved or eliminated. Middle left: Survivorship variance held at biological value, and recruitment variance takes the biological value, halved or eliminated. Bottom left: Variability of survivorship and recruitment vary from both biological, to half biological and variability in both removed. Right 3 panels: 10 randomized abundance simulations with the variability in survivorship (Svar) and recruitment (Rvar) biological, both half biological, and both removed (0). All scenarios have mean recruitment of 0.30 and mean survivorship of 0.90 (stochastic λ = 1.02).
Examination of demographic parameters
We determined the effects of temporal and geographical (across population) variability. Given 9–10 consecutive surveys, mean recruitment and biological variance were 0.30 and 0.028 ( n = 11; CV = 0.56), matching results from all these recruitment data. Within a 5 year consecutive period, recruitment = 0.31 with variance = 0.021 ( n = 24; CV = 0.48). For 3 year consecutive periods, the mean recruitment ratio = 0.29 with variance = 0.018 ( n = 73; CV = 0.46). Reducing data duration lowered the recruitment variance. Mean values remained consistent.
We examined geographical variation in recruitment data (L:EY) with populations ≥ 20 adult females and ≥ 10 years ( n = 32), generating a mean L:EY of 0.29. The mean and standard deviation of the variance on these data (i.e., average and standard deviation of the individual variance values) was 0.024 and 0.015, respectively. Hence, there is considerable variability in the variance of L:EY across populations.
Most survivorship data we use originated from desert bighorn sheep within the Peninsular Ranges of California spanning 4–5 years 22 , with CV = 0.14. CVs of survivorships from these populations span 0.10 through 0.19, and CVs for the first 3 years of data average 14% lower than the CV from all years (SD = 0.27). Variation in survivorship also appears geographical and temporal.
The magnitude of change in λ when recruitment shifts from 0.20 to 0.40 and survivorship remains fixed at 0.90 is 0.17. The magnitude of change in λ when survivorship moves from 0.75 to 0.95 and recruitment remains fixed at 0.30 is 0.17. Similar, additive changes in adult female survivorship and recruitment generated comparable results in λ.
Adult female translocation
Given a hypothetical removal of 5 adult females during each of 5 successive years (years 1–5), a population of 50 adult females declined to a mean low of 30 (90% CI 6–62) in year 5, and returned to 50 by year 27 (90% CI 0–173). The QE (10) is 0.05 and QE (30) 0.18, and the lower confidence interval for abundance hits 0 by year 18 (Fig. 4 ). Populations < 50 did not return to the initial abundance value within 30 years. A population must have a minimum starting abundance of 70 adult females to avoid the 90% lower confidence interval reaching 0. This population attained a low of 52 (90% CI 17–100) in year 5, and returned to 70 by year 17 (90% CI = 8–192; Fig. 4 ), with QE (10) 0.01 and QE (30) 0.07.
Abundance trajectories of adult females, with removals of 5 adult females during each of the first 5 consecutive years (left), and the removal of 10 adult females during each of the first 3 consecutive years (right). Mean abundances are solid lines with dashed lines representing 90% confidence intervals. Given these 5 or 10 removals, starting abundance must be ≥ 70 adult females for the population to return to a mean of 70 within 30 years without the lower confidence interval reaching 0. Inset values indicate the starting abundances of adult females.
A population of 50 adult females with 10 removals per year for 3 consecutive years, declined to 23 by year 4 (90% CI 6–47), and returned to 44 by year 30 (90% CI 0–152; Fig. 4 ). A population requires a starting abundance of 60 adult females to return to 60 by year 28, although the lower confidence interval reached 0 by year 24. Again, a population required a starting abundance of 70 adult females for the lower confidence interval to never attain 0. This hypothetical population reached a low of 46 (90% CI 17–82) in year 4, returned to 70 (90% CI 5–210) by year 22 (Fig. 4 ), with QE (10) 0.01 and QE (30) 0.08.
Given Caprinae life history and plausible combinations of mean recruitment and adult female survivorship, we evaluated population persistence and estimated population MVP. The values describing adult female survivorship and recruitment, plus the variability we employed match values found in other populations of Caprinae . We do not pool data across different Caprinae populations or species. Our approach and results directly inform the conservation and management of many Caprinae , especially those for which the acquisition of demographic data remains beyond reach.
Our work embodies the characteristics of a high-quality PVA: clear objectives, appropriate demographic data, model structure matching species life histories, stochasticity, examination of extinction probability, appropriate time interval, use of mean values and associated variability 6 . As with most ecological models, the quest for more data remains problematic, not debilitating, and is addressed by creatively and aptly using existing information to generate meaningful results 3 .
Wildlife agencies generate lamb:adult female ratios from Caprinae surveys, recognizing that yearlings can be mistaken for adult females, causing miscounts. Excluding yearlings from the ratio’s denominator assumes that no miscounts are occurring, yet an unknown and inconsistent number of yearlings remain in the adult female category across survey events. For these reasons, surveyors of other species, like Dall’s sheep and caribou, pool counts of yearlings and adult females, generating lamb:“adult female-like” ratios instead 15 , 23 , 24 , 25 .
Managers of Caprinae populations can follow these precedents and produce lamb:(adult female + yearling) ratios. Consistency would help standardize methods for building comparisons and meta-analyses across populations of Caprinae , while reducing variability across surveys due to differing techniques.
Typically, metrics like elasticity (proportional) and sensitivity (additive) describe the influences of demographic parameters on population growth 13 , 14 , 22 , 26 . For Caprinae , when adult female survivorship is 0.90 and recruitment 0.30, the elasticity in survivorship and recruitment are 0.61 (90% CIs 0.40–0.75) and 0.24 (90% CIs 0.13–0.40) respectively (elasticity in young adult survivorship is 0.16 (90% CIs 0.12–0.21). For ungulates in general, the elasticity values for survival tend to be higher than those for recruitment 27 . Our results match this pattern, as the elasticity results indicate that a change in adult survival has a 2.5 times greater effect on λ than an equivalent change in recruitment. Relatedly, other theoretical work reports that demographic parameters with more temporal variability have lower elasticities, indicating less impact on population fitness (e.g. 28 , 29 ).
Our work centers on applications. Since most management actions affect these demographic parameters simultaneously, at issue is the practicality (e.g. feasibility and affordability) of management to increase these parameters, and understanding how such changes could impact λ. For example, imagine a population with mean recruitment of 0.30 and adult survival 0.85, with a biologist interested in increasing recruitment or adult female survival to acquire λ ≥ 1. The answer is to increase either value by 0.02 (Fig. 1 , Supplementary Data S1 ). Similarly, one can set a λ target and determine the amount of recruitment and adult female survival necessary for acquiring it (Fig. 1 , Supplementary Data S1 ).
Minimum abundance target
A minimum population of 50 adult females meets the persistence criteria, given intermediate levels of recruitment and survival producing λ ~ 1 (Table 2 ). The risk of population collapse wanes as populations increase above the minimum threshold (Table 2 ; Fig. 1 ). For example, a population of ~ 100 adult females always meets persistence criteria (Table 2 ). Populations of adult females should be somewhat larger than 50 when modest declines (λ ~ 0.97) are suspected, providing a cushion to address the causes of decline, and mitigate further reductions.
Translocation of 5 adult females during each of 5 years, or 10 in each of 3 years, requires a starting abundance of 70 adult females for the population to maintain the persistence criteria, never reach a lower confidence interval of 0, and for the population to return to the starting population size within 30 years. If managers mistakenly target a population having < 50 adult females, the population mean is unlikely to recover to pre-removal levels within 30 years (Fig. 4 ). The more adult females removed per year will reduce a populations abundance and elevate stochastic effects.
Applications
These survival and recruitment parameters have high temporal and geographical variability. This uncertainty originates from the variability inherent to survivorship and recruitment data within Caprinae populations and demographic stochasticity operating over all age classes, making it difficult to predict exact values of λ and project abundances (Fig. 3 ). The causes of such variability are complex (e.g. factors such as variation in environmental drivers, predation, management types and timing, the distribution of female reproduction across populations and ages through time), and are rarely monitored. Without data, these factors cannot be explicitly examined. The effects of these factors, however, are contained in the empirically-derived estimates for survival and recruitment, and their associated variability.
We examined the effects of time and management actions on reducing variation in recruitment and adult female survivorship. We began by halving the variance in recruitment, given its empirical basis. For all recruitment data, when the temporal window is short (3 years), recruitment variability declined to nearly half the overall mean variability. Also, the NMDGF operate a fenced facility (6.2 km 2 ) for desert bighorn sheep, to translocate surplus animals elsewhere. These sheep remain wild, yet receive year-round water and rare predation events. This population has biological variance in recruitment approximately half that of the variance of other populations of desert bighorn in the southwest U.S. (variance = 0.015). The mean recruitment value (L:EY) recorded within this facility (0.56; N = 18 years; CV = 0.22) is nearly double the amount of recruitment calculated from the other populations of desert bighorn sheep.
For survival, most of these data represented desert bighorn sheep populations whose abundance were declining (disease, predation, habitat loss 22 ). Survivorship of adult females in endangered or declining populations of ungulates could be lower or more variable than in demographically viable populations 30 , 31 , 32 .
Management actions can reduce variation in recruitment and survival. However, the simulations halving variance in recruitment or survival received minimal increases in precision in λ or abundance (Table 4 ; Fig. 3 ). Halving variability in adult female survivorship and recruitment, or eliminating it, moved the probability of λ < 1 to 0.39 and 0.31 (from 0.41) while reducing QE (30) from 0.05 to 0.01 and 0 respectively (Table 4 ). Therefore, in application, halving variance in recruitment (i.e., calculated from predator-free populations) and survival (i.e., from predator control) does not generate much change in population trajectories.
Management actions can also change the mean values of demographic parameters. For instance, populations of grazing mammals lacking predation have higher growth rates with less variability 33 . Therefore, when populations experience predation, predator control can improve population sizes of Caprinae by raising the survival of adult females and recruitment 12 , 34 , 35 . Survival of adult females can increase 5–12% with predator control, and survival with predation is often twice as variable as populations without predation 12 . Imagine predator control boosted mean adult female survival from 0.90 to 0.95 12 and recruitment from 0.30 to 0.50 (similar to the NM penned facility), while reducing the survival and recruitment variance by half ( 12 ; NM penned facility). Population growth would increase 20%, from λ = 1.02 to 1.22. Mean abundance would climb from 56 adult females to 135 by year 5.
Predators are important components of ecosystem health 36 , 37 , so predator management requires conscientious approaches. Predator control (or perhaps reduction in conspecific, alternative prey) could be applied to improve Caprinae populations with < 50 adult females. Larger populations should withstand background levels of predation, when stable or growing. Curbing declines in larger populations, or attaining targets in population growth or abundances could also warrant predator control, to accomplish management objectives. Monitoring the abundance and recruitment of Caprinae populations helps identify conditions suggesting management actions like predator control, while indicating when the desired project objectives are achieved (i.e. Caprinae population responses). Factors like environment, climate and disease influence population abundance too, and are worth considering during such monitoring 38 .
Provision of supplemental water can increase the distributions of Caprinae 39 , 40 . Supplemental water, however, could increase predator distributions (directly or indirectly), potentially aggravating predation issues affecting adult female survival and recruitment 41 , 42 . Other hypotheses, like temporal reductions in water supplementation, may also reduce predator presence within Caprinae ranges 43 , 44 . Alternative management tools for improving Caprinae abundances are supplemental feeding and disease control, challenging and costly to pursue at large scales.
The variability in adult female survival and recruitment means that 1 year’s recruitment or survival cannot accurately predict the next, and historical trends in population abundance may have little bearing on future performance (Fig. 3 ). Revealing management contributions to population growth is also challenged by the variability inherent within the demographics of Caprinae populations. This situation makes Caprinae management reactive, with management decisions based on the short-term monitoring results for a population. The repeated acquisition of accurate abundance data, however, builds a more proactive paradigm.
Monitoring Caprinae
Population vital rates and their importance to population growth often varies across populations and within a population over time (our results 31 , 32 , 45 , 46 ). Indeed, within and across populations of Caprinae, the annual differences in survival and recruitment are substantial 47 , 48 . Some temporal and geographical variation is biological, and some stems from the use of different methodologies for quantifying variability. Hence, analytical projections of future abundance based on survival and recruitment data from one population are unlikely to apply to other populations for the same or different species. Understanding a given populations status requires directly monitoring that population.
Frequent monitoring of many populations requires simple and affordable methods for identifying a population’s status (abundance, growth). We discovered the threshold of 50 adult females with three types of demographic parameters: abundance, survival and recruitment. Monitoring the threshold is achievable with two of them.
Adult female survival is the most challenging and costly parameter to acquire, unattainable for most biologists managing Caprinae populations. Indeed, we struggled to locate data describing adult female survival given a species extensively studied. Future monitoring and analyses, therefore, do not require survival data, but can rely solely on measures of abundance and recruitment.
Minimum counts are the primary method for monitoring Caprinae species. This method is appropriate if precision measurements are not required and acknowledgement that data are biased low. With minimum counts, management actions dependent upon abundance triggers (i.e., 50 adult females) would be identified early, providing a conservative approach to population management. In the short term, populations can be prioritized for conservation using minimum abundances, but these data make it difficult to generate longer-term conclusions about population sustainability.
When minimum counts or mean abundance estimates for adult females are well above 50 (i.e. ~ ≥ 75), the population is likely to persist (unless conditions change). When populations occur at or near the threshold value, recruitment estimates associated with these abundance estimates (and trends) help biologists infer if the population is stable or growing, and if the minimum abundance threshold were reached. For example, imagine a population with known abundance of 70 adult females. A survey was conducted, producing an estimate of 65 adult females (90% CI 44–86 (20% CV)), with a recruitment estimate (Lamb:(adult female + yearling)) > 0.30. Trends in abundance from prior surveys indicate an increase. A reasonable interpretation of these data is that the population is likely at or above the threshold and growing.
We used abundance trends because of the high variability in recruitment trends (demonstrated herein). If either abundance, abundance trend or recruitment shows a concerning decline, it should initiate work to discern the cause, as the population may no longer be sustainable.
While the acquisition of accurate abundance data can be challenging 48 , 49 , 50 , researchers have posed tenable solutions for meeting these challenges 50 , 51 . These methods rely on methodological changes in aerial surveys, or replacing aerial surveys with motion activated cameras, that collaterally identify predators and their relative abundances.
For annually-reproducing Caprinae that begin reproduction in their second to third year, the minimum abundance threshold of 50 adult females should apply. Source populations for translocation have a 70 adult female abundance threshold, assuming translocations of 5 or 10 adult females per year, in each of 5 and 3 years respectively. For Caprinae with different life histories, our model code is easily modified to accommodate those changes.
Hence, our work extends beyond Caprinae . The population projection matrix model we developed can be applied to any species reproducing on an annual cycle. Users specify parameters such as the number of stage-classes, mean and variance of recruitment and adult survival, starting population size, correlation between recruitment and survival, sex-ratio at recruitment, and number of translocations (Supplementary Data S1 ). As exemplified herein, results will identify a population's’minimum threshold for persistence, which subsequently informs threat assessments, harvest quotas and the triage of conservation activities aimed at recovering ailing populations.
Our matrix model simulated population trajectories given various combinations of starting abundances, recruitment, adult female survival values, and translocation scenarios (Fig. 5 ). We predicted population growth, adult female abundance and determined extinction and quasi-extinction ( QE) probabilities through time. We defined extinction when abundance = 0 adult females, and QE when abundance ≤ 5 adult females. Results from these simulations enabled us to identify a minimum target abundance of Caprinae to ensure population persistence 4 . We defined persistence as a population with ≤ 0.01 chance of QE in 10 years and ≤ 0.10 chance of QE in 30 years.
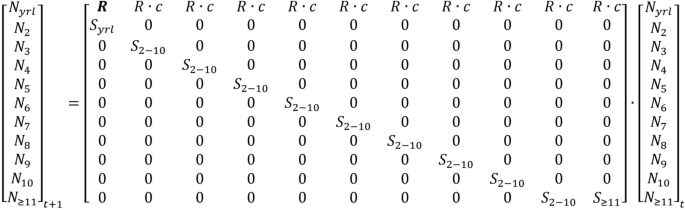
Population projection matrix used for simulating Caprinae population growth and the abundance of adult females. The first recruitment entry (bold R) is a necessary computational step to correctly represent reproduction, and account for the observer misclassifications of yearling male and females as adult females. Symbols indicate the following: R = recruitment, S = adult female survivorship, c = 0.5 (sex ratio), N = Age class.
We examined peer-reviewed publications by searching “Google Scholar” for mean recruitment and survival parameters reported for Caprinae species, to ensure that model parameters correspond with data representing a diversity of Caprinae . Our searches covered all years, and included ≥ 1of the following keywords: Abundance, Ammotragus, argali, Capra, Capricornus, Caprinae, Caprini, chamois, goat, goral, ibex, mouflon, Naemorhedus, Oreamnos, Ovibovini, Ovis, recruitment, Rupicapra, Rupicraprini, serow, sheep, survival, survivorship, takin, tur, urial. The simulations and analyses also required an accurate measure of biological variance in Caprinae recruitment and adult female survival to ensure that robust measures of variability accompanied the resulting predictions. Recruitment and survivorship surveys often differ in their methodology (e.g. aerial, ground counts, telemetry), timing and duration. To ensure a robust understanding of the variability in recruitment and survivorship data entering the simulations, we avoided arbitrarily selecting values from the literature. Instead, we calculated the variability in these parameters from data representing desert bighorn sheep. We compared the variability values gained from our calculations to those presented for other Caprinae to ensure relevance and transferability in results.
We found few publications describing ewe survival for Caprinae . Therefore, to compute variability, we relied on survival data for desert bighorn sheep from three publications with studies spanning multiple, sequential years 22 , 52 , 53 . To calculate variability in recruitment, we acquired recruitment data on desert bighorn sheep from three state agencies: Arizona Game and Fish Department (AZGFD; 1958–2008; 90 locations), California Department of Fish and Wildlife (CDFW; 1991–2010; 13 locations), and New Mexico Department of Game and Fish (NMDGF; 1979–2016; 6 locations). Most Caprinae bear one young per year (periodic twinning can occur; an outcome more common in some species), with females reproducing during their second or third year until death 1 . Desert bighorn sheep share these characteristics. We estimated the biological variance (i.e., the component of variance due to temporal and environmental variation) of survival and recruitment by using the variance discounting procedures which removes the sampling variation 54 .
The stage-structured population projection matrix model used 11 age-based categories (Fig. 5 ). Our models used age-class delineations corresponding to a post-breeding census. We considered lambs (or kids) < 0.5 years, yearlings as ages ≥ 0.5 and < 1.5 years, and adult females as ≥ 1.5 and ≤ 12.5 years (herein, the term “lamb” is also interchangeable for “kid”, when the sentence is used in context for Caprinae species). We set yearling survivorship as 10% lower than adult female survivorship, a reduction based on prior work with bighorn sheep 55 . We considered prime aged animals ages 2–10 years, with survival equivalent across these age classes 55 , 56 . Older females (≥ 11.5) received a 0.43 proportional reduction in survival from adult female survival (calculated from life history table 56 ). We set the amount of variation in yearling and older female survival to equal the variation in adult survival.
We integrated demographic stochasticity (inherent randomness that arises from the discrete nature of individual animals 57 ) and biological variation into the simulation. Biological variation was derived from the empirical recruitment and survival data. For each year and iteration, we randomly selected adult female survival and lamb recruitment using the incomplete beta function, which allows for correlated vital rates 58 . We assumed the vital rates were correlated at 0.40 (calculated from 22 ). We used the first and second moments to calculate the scale and shape parameters of the beta distribution 58 , 59 . We assumed a 50 male:50 female sex ratio of recruited lambs, normally distributed with a variance of 0.01 60 . We incorporated demographic stochasticity as a binomial random variable through the random generation of survivors and recruits. The number of trials ( n ) matched the number of individual adult females in a given stage, with the parameter p of the binomial distribution a random variable simulated from the beta distribution.
Our simulations used combinations of mean survival and recruitment values to estimate population growth (λ). We allowed mean survival to range from 0.70 to 0.98, mean recruitment from 0.05 to 0.90 (to account for scenarios with and without twinning) and starting abundance from 10 to 250 adult females. Biological variance remained fixed to empirical values. We explored the effects of biological variation on population dynamics in subsequent simulations by artificially halving and eliminating the recruitment and survivorship variances. We also identified how changes in survivorship and recruitment affected λ.
While multiple factors affect the mortality of adult females (e.g. predation, starvation, disease), declines in abundance also occurs when adult females are translocated from a source population to another population. Translocations of adult females should be an acceptable practice, provided the source population does not encumber a high risk of QE resulting from removals. When desert bighorn sheep are translocated from a population, it is common for managers to pursue sequential years of removals. Therefore, we examined the effects of adult female removals on population stability by examining two translocation scenarios: removing 5 adult females consecutively for 5 years (25 total removals) and removing 10 adult females over 3 consecutive years (30 total removals). These translocation simulations fixed recruitment and survival parameters to generate a stochastic λ = 1.02 (recruitment = 0.30, survivorship = 0.90, with biological variability), as populations with λ ≤ 1.00 would not recover from the translocation event.
Simulations estimated abundance and λ for each combination of survival and recruitment, every year, for 30 years, with 10,000 individual iterations. We tested model operation to ensure results replicated the values of mean recruitment, mean survival and their respective variability. For each simulation we determined extinction and QE each year.
Data describing lamb:adult female ratios typically represent recruitment, a combination of adult female fecundity and lamb survival. Lambs are born in spring and surviving lambs are counted and considered recruited during fall surveys (~ 6 months post-birthing). Our model relies on recruitment ratios, as these data are routinely collected by Caprinae biologists. For Caprinae , age-specific recruitment data are rarely (if ever) recorded during population monitoring. The variability in the lamb:ewe data accounts for any differences in reproduction across age classes through time and across populations.
Management agencies report lamb:adult female ratios differently, with some agencies including or excluding yearlings in the ratio denominator. We examined these lamb:adult female data, finding that yearlings (of both sexes) were often misclassified as adult females. This outcome is not unusual, as surveys for other species often misclassify yearling male and females as adult females 25 . Grouping yearlings and adult females into a ratio of lamb:‘adult female-like’ is also a common solution, for representing recruitment in Caprinae and other ungulates 15 , 24 , 61 . Therefore, we employed lamb:‘adult female-like’ ratios in the simulations.
Mathematically, we adjusted the population projection matrix to account for the low bias in recruitment, occurring from the inclusion of yearling males and females in the denominator of the ratio. This simple adjustment multiplied the recruitment ratio by 2 in the first column of the matrix (bold R representing the first age category; Fig. 5 ; Supplementary Data S1 ). To explain, imagine a hypothetical population consisting of 80 adult females (E) producing 30 lambs (L), with 10 male and 10 female yearlings (YM and YF respectively). The conventional lamb:adult female ratio (L:E) is 0.375. An unknown number of yearlings, however, are misclassified as adult females. When all yearlings are included in the denominator of the lamb:adult female ratio (L:(E + YM + YF)) the recruitment ratio is biased low (0.30). The traditional matrix would only include R in the 2nd–11th column of the matrix (the bold R omitted in the matrix within Fig. 5 ), which would produce 24 lambs (80 × 0.30 = 24). Yet we know from the example, that 30 lambs were produced. Alternatively, if R occurred in all columns of the matrix, the result is 27 lambs (80 × 0.30 + 10 × 0.30 = 27) and lamb production remains biased low. This problem arises because male and female yearlings are not reliably distinguished. To correct this problem, we multiplied R by 2 in the first column (80 × 0.30 + 10 × 2 × 0.30 = 30), which accounts for yearling males. The 30 lambs includes both sexes, and since our simulation represents adult females, R is multiplied by the lamb sex ratio (c = 0.5).
Data availability
Program and sample data to run analyses are included.
Shackleton, D. M. (ed.) and the IUCN/SSC Capriane Specialist Group. Wild Sheep and Goats and Their Relatives. Status Survey and Conservation Action Plan for Caprinae (IUCN, 1997).
Ovaskainen, O. & Meerson, B. Stochastic models of population extinction. Trends Ecol. Evol. 25 (11), 643–652 (2010).
Article PubMed Google Scholar
Burgman, M. & Possingham, H. Population viability analysis for conservation: The good, the bad and the undescribed. In Genetics, Demography and Viability of Fragmented Populations (eds Young, A. G. & Clarke, G. M.) 97–112 (Cambridge University Press, 2000).
Chapter Google Scholar
Pe’er, G. et al. A protocol for better design, application, and communication of population viability analyses. Conserv. Biol. 27 (4), 644–656 (2013).
Ralls, K., Beissinger, S. R. & Cochrane, J. F. Guidelines for using population viability analysis in endangered-species management. In Population Viability Analysis (eds Beissinger, S. R. & McCullough, D. R.) 521–550 (University of Chicago Press, 2002).
Google Scholar
Chaudhary, V. & Oli, M. K. A critical appraisal of population viability analysis. Conserv. Biol. 34 (1), 26–40 (2020).
Caswell, H. Matrix Population Models Vol. 1 (Sinauer Associates, Sunderland, 2000).
Mills, L. S. Conservation of Wildlife Populations: Demography, Genetics, and Management (Blackwell Publishing, 2007).
Grenier, M. B., McDonald, D. B. & Buskirk, S. W. Rapid population growth of a critically endangered carnivore. Science 317 (5839), 779–779 (2007).
Article ADS CAS PubMed Google Scholar
Enneson, J. J. & Litzgus, J. D. Using long-term data and a stage-classified matrix to assess conservation strategies for an endangered turtle ( Clemmys guttata ). Biol. Conserv. 141 (6), 1560–1568 (2008).
Article Google Scholar
Beissinger, S. R. & Westphal, M. I. On the use of demographic models of population viability in endangered species management. J. Wildl. Manag. 63 (3), 821–841 (1998).
Festa-Bianchet, M., Coulson, T., Gaillard, J. M., Hogg, J. T. & Pelletier, F. Stochastic predation events and population persistence in bighorn sheep. Proc. R. Soc. B Biol. Sci. 273 (1593), 1537–1543 (2006).
Gaillard, J. M., Festa-Bianchet, M. & Yoccoz, N. G. Population dynamics of large herbivores: Variable recruitment with constant adult survival. Trends Ecol. Evol. 13 (2), 58–63 (1998).
Article CAS PubMed Google Scholar
Gaillard, J.-M., Festa-Bianchet, M., Yoccoz, N. G., Loison, A. & Toïgo, C. Temporal variation in fitness components and population dynamics of large herbivores. Annu. Rev. Ecol. Syst. 31 , 367–393 (2000).
Rattenbury, K. L. et al. Delayed spring onset drives declines in abundance and recruitment in a mountain ungulate. Ecosphere 9 (11), e02513 (2018).
Festa-Bianchet, M., Urquhart, M. & Smith, K. G. Mountain goat recruitment: Kid production and survival to breeding age. Can. J. Zool. 72 (1), 22–27 (1994).
Awan, G. A., Festa-Bianchet, M. & Ahmad, T. Poaching, recruitment and conservation of Punjab urial Ovis vignei punjabiensis . Wildl. Biol. 12 (4), 443–444 (2006).
Haider, J., Rakha, B. A., Anwar, M., Khan, M. Z. & Ali, H. An updated population status of Astor Markhor ( Capra falconeri falconeri ) in Gilgit-Baltistan, Pakistan. Glob. Ecol. Conserv. 27 , e01555 (2021).
Clutton-Brock, T. H., Price, O. F., Albon, S. D. & Jewell, P. A. Early development and population fluctuations in Soay sheep. J. Anim. Ecol. 61 , 381–396 (1992).
Cransac, N., Hewison, A. J. M., Maublanc, M. L., Gaillard, J. M. & Cugnasse, J. M. Patterns of mouflon ( Ovis gmelini ) survival under moderate environmental conditions: Effects of sex, age, and epizootics. Can. J. Zool. 75 (11), 1867–1875 (1997).
Hoefs, M. & Bayer, M. Demographic characteristics of an unhunted Dall’s sheep ( Ovis dalli dalli ) population in southwest Yukon, Canada. Can J. Zool. 61 (6), 1346–1357 (1983).
Rubin, E. S., Boyce, W. M. & Caswell-Chen, E. P. Modeling demographic processes in an endangered population of bighorn sheep. J. Wildl. Manag. 66 , 796–810 (2002).
DeCesare, N. J. et al. Estimating ungulate recruitment and growth rates using age ratios. J. Wildl. Manag. 76 (1), 144–153 (2012).
van de Kerk, M., Verbyla, D., Nolin, A. W., Sivy, K. J. & Prugh, L. R. Range-wide variation in the effect of spring snow phenology on Dall’s sheep population dynamics. Environ. Res. Lett. 13 (7), 075008 (2018).
Article ADS Google Scholar
Smith, B. L. & McDonald, T. L. Criteria to improve age classification of antlerless elk. Wildl. Soc. Bull. 30 , 200–207 (2002).
De Kroon, H., Van Groenendael, J. & Ehrlén, J. Elasticities: A review of methods and model limitations. Ecology 81 (3), 607–618 (2000).
Heppell, S. S., Caswell, H. & Crowder, L. B. Life histories and elasticity patterns: Perturbation analysis for species with minimal demographic data. Ecology 81 (3), 654–665 (2000).
Pfister, C. A. Patterns of variance in stage-structured populations: Evolutionary predictions and ecological implications. Proc. Natl. Acad. Sci. 95 (1), 213–218 (1998).
Article ADS CAS PubMed PubMed Central Google Scholar
Hilde, C. H. et al. The demographic buffering hypothesis: Evidence and challenges. Trends Ecol. Evol. 35 (6), 523–538 (2020).
Owen-Smith, N. & Mason, D. R. Comparative changes in adult vs. juvenile survival affecting population trends of African ungulates. J. Anim. Ecol. 74 (4), 762–773 (2005).
Nilsen, E. B. et al. A slow life in hell or a fast life in heaven: Demographic analyses of contrasting roe deer populations. J. Anim. Ecol. 78 (3), 585–594 (2009).
Johnson, H. E., Mills, L. S., Stephenson, T. R. & Wehausen, J. D. Population-specific vital rate contributions influence management of an endangered ungulate. Ecol. Appl. 20 (6), 1753–1765 (2010).
Van Houtan, K. S., Halley, J. M., Van Aarde, R. & Pimm, S. L. Achieving success with small, translocated mammal populations. Conserv. Lett. 2 (6), 254–262 (2009).
Ballard, W. B., Whitman, J. S. & Reed, D. J. Population dynamics of moose in south-central Alaska. Wildl. Monogr. 114 , 3–49 (1991).
Rominger, E. M. The Gordian knot of mountain lion predation and bighorn sheep. J. Wildl. Manag. 82 (1), 19–31 (2018).
Sinclair, A. R. E., Mduma, S. & Brashares, J. S. Patterns of predation in a diverse predator–prey system. Nature 425 (6955), 288–290 (2003).
Beschta, R. L. & Ripple, W. J. Large predators and trophic cascades in terrestrial ecosystems of the western United States. Biol. Conserv. 142 (11), 2401–2414 (2009).
Sæther, B. E. Environmental stochasticity and population dynamics of large herbivores: A search for mechanisms. Trends Ecol. Evol. 12 (4), 143–149 (1997).
Krausman, P. R., Leopold, B. D., Seegmiller, R. F. & Torres, S. G. Relationships between desert bighorn sheep and habitat in western Arizona. Wildl. Monogr. 102 , 3–66 (1989).
Krausman, P. R., Rosenstock, S. S. & Cain, J. W. III. Developed waters for wildlife: Science, perception, values, and controversy. Wildl. Soc. Bull. 34 (3), 563–569 (2006).
Harrington, R. et al. Establishing the causes of the roan antelope decline in the Kruger National Park, South Africa. Biol. Conserv. 90 , 69–78 (1999).
Owen-Smith, N. Ecological guidelines for waterpoints in extensive protected areas. S. Afr. J. Wildl. Res. 26 (4), 107–112 (1996).
Harris, G., Sanderson, J. G., Erz, J., Lehnen, S. E. & Butler, M. J. Weather and prey predict mammals’ visitation to water. PLoS One 10 (11), e0141355 (2015).
Article PubMed PubMed Central CAS Google Scholar
Harris, G. M. et al. Year-round water management for desert bighorn sheep corresponds with visits by predators not bighorn sheep. PLoS One 15 (11), e0241131 (2020).
Article CAS PubMed PubMed Central Google Scholar
Albon, S. D. et al. Temporal changes in key factors and key age groups influencing the population dynamics of female red deer. J. Anim. Ecol. 69 (6), 1099–1110 (2000).
Morrison, S. F. & Hik, D. S. Demographic analysis of a declining pika Ochotona collaris population: Linking survival to broad-scale climate patterns via spring snowmelt patterns. J. Anim. Ecol. 76 (5), 899–907 (2007).
Côté, S. D., Festa-Bianchet, M. & Smith, K. G. Compensatory reproduction in harvested mountain goat populations: A word of caution. Wildl. Soc. Bull. 29 (2), 726–730 (2001).
Hamel, S., Côté, S. D., Smith, K. G. & Festa-Bianchet, M. Population dynamics and harvest potential of mountain goat herds in Alberta. J. Widl. Manag. 70 (4), 1044–1053 (2006).
Gonzalez-Voyer, A., Festa-Bianchet, M. & Smith, K. G. Efficiency of aerial surveys of mountain goats. Wildl. Soc. Bull. 29 , 140–144 (2001).
Conroy, M. J., Harris, G., Stewart, D. R. & Butler, M. J. Evaluation of desert bighorn sheep abundance surveys, southwestern Arizona, USA. J. Wildl. Manag. 82 (6), 1149–1160 (2018).
Harris, G. M., Butler, M. J., Stewart, D. R., Rominger, E. M. & Ruhl, C. Q. Accurate population estimation of Caprinae using camera traps and distance sampling. Sci. Rep. 10 (1), 1–7 (2020).
Article CAS Google Scholar
Cunningham, S. C. & DeVos, J. C. Mortality of mountain sheep in the Black Canyon area of northwest Arizona. Des. Bighorn. C. Trans. 36 , 27–29 (1992).
Overstreet, M. W. Body condition and survival of desert bighorn sheep in relation to habitat and precipitation on the Kofa National Wildlife Refuge, Arizona (Doctoral dissertation, New Mexico State University, 2014).
White, G. C. Population viability analysis: Data requirements and essential analyses. In Research Techniques in Animal Ecology: Controversies and Consequences (eds Boitani, L. & Fuller, T. K.) 288–331 (Columbia University Press, 2000).
Jorgenson, J. T., Festa-Bianchet, M., Gaillard, J. M. & Wishart, W. D. Effects of age, sex, disease, and density on survival of bighorn sheep. Ecology 78 (4), 1019–1032 (1997).
Hansen, C. G. Bighorn sheep populations of the Desert Game Range. J. Wildl. Manag. 31 , 693–706 (1967).
Boettiger, C. From noise to knowledge: How randomness generates novel phenomena and reveals information. Ecol. Lett. 21 , 1255–1267 (2018).
Morris, W. F. & Doak, D. F. Quantitative Conservation Biology: Theory and Practice of Population Viability Analysis (Sinauer Associates, 2002).
Casella, G. & Berger, R. L. Statistical Inference (Cengage Learning, 2002).
MATH Google Scholar
DeVore, R. M., Butler, M. J., Wallace, M. C. & Liley, S. G. Population dynamics model to inform harvest management of a small elk herd in central New Mexico. J. Wildl. Manag. 9 , 531–544 (2018).
Hayes, R. D. et al. Experimental reduction of wolves in the Yukon: Ungulate responses and management implications. Wildl. Monogr. 152 , 1–35 (2003).
Download references
Acknowledgements
We thank E. Rominger and J. Gedir for project ideas and assistance, and AZGFD, NMDGF and CAFW for data. Reviews by M. Conroy, E. Goldstein and two anonymous reviewers improved an earlier draft of this manuscript. The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views of the U.S. Fish and Wildlife Service. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and affiliations.
United States Fish and Wildlife Service, Albuquerque, NM, USA
Grant M. Harris, Matthew J. Butler & David R. Stewart
US Geological Survey New Mexico Cooperative Fish and Wildlife Research Unit, Las Cruces, NM, USA
James W. Cain III
You can also search for this author in PubMed Google Scholar
Contributions
G.M.H. made substantial contributions to the conception and design of the work, the acquisition, analysis, and interpretation of data, plus manuscript writing. M.J.B. made substantial contributions to the conception and design of the work, the acquisition, analysis, and interpretation of data, plus manuscript writing. D.R.S. made substantial contributions to the conception and design of the work, the acquisition, analysis, and interpretation of data, plus manuscript writing. J.W.C. made substantial contributions to the conception and design of the work, the acquisition, analysis, and interpretation of data, plus manuscript writing.
Corresponding author
Correspondence to Grant M. Harris .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Supplementary Information
Supplementary information 1., supplementary information 2., supplementary legends., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Harris, G.M., Butler, M.J., Stewart, D.R. et al. The abundance and persistence of Caprinae populations. Sci Rep 12 , 13807 (2022). https://doi.org/10.1038/s41598-022-17963-w
Download citation
Received : 08 October 2021
Accepted : 03 August 2022
Published : 15 August 2022
DOI : https://doi.org/10.1038/s41598-022-17963-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Scientific Name
Location in taxonomic tree, identification numbers.
- Destinations Istanbul Mediterranean
- Destinations Antalya
- Izmir is Turkeys third largest city and the

Company Line
0242 322 37 70
- Destinations Cappadocia

Cappadocia, located in Central Anatolia, was named after Cappodocians who settled there about 700 B.C., when craters and chimneys dominated the landscapes. Then million years ago, mount Erciyes erupted violently and Mount Hasan covered the ground with tufa, a soft Stone made from lava, ash and mud. Then the rain, wind and flood waters from the Kızılırmak River shaped the tufa into the most mysterious remarkable cone shaped structures called peribaca "fairy chimneys". Red sandstone and salt deposits of the Miocene period characterize the sparsely inhabited landscape. The relatively small areas of the fertile soil on volcanic tuff are where the population tends to migrate. The southern part of Cappadocia is often spoken of as the heart of the region. Cappadocia is best known for potatoes, fruits ans wine.
One of Cappadocia’s most interesting villages is found below the town of Derinkuyu, the underground city. This town dates back to the Hittite times, but evidence from excavations revealed that the Hittites only used the top level. Currently there have been discovered eight levels of dwellings, storerııms, chapels and parts of monasteries. These man made underground caves are grouped around a 280 feet shaft with a cleverly devised ventilation system consisting of 52 vents. Other underground passages linked the city with other cities in the communities. At the center of the city is a chapel, which today is used as a mosque. It dates back to the 16th or 17th century and contains paintings of jesus, Mary, angels and saints.
"Goreme" the famous rock churches lies adjacent to the village of Goreme. Half this village consist of cave dwellings. Numerous rock churches can be found in this area as well as the spectacular "open air museum". Before 1964 these churches were not propected but now are protected by tour coverings on various sights. Other sites include the Dervent, Zelve, Ihlara, and Soganli Valleys as well as the city of Uchisar.
Cappadocia’s charming landscape remains unparalleled in history and mystery.
© 2024 Tüm hakları saklıdır. Reklamlarım Yazılım Hizmetleri

- Visit Our Blog about Russia to know more about Russian sights, history
- Check out our Russian cities and regions guides
- Follow us on Twitter and Facebook to better understand Russia
- Info about getting Russian visa , the main airports , how to rent an apartment
- Our Expert answers your questions about Russia, some tips about sending flowers

Russian regions
- Arkhangelsk oblast
- Kaliningrad oblast
- Karelia republic
- Komi republic
- Leningrad oblast
- Murmansk oblast
- Nenets okrug
- Novgorod oblast
- Pskov oblast
- Vologda oblast
- Map of Russia
- All cities and regions
- Blog about Russia
- News from Russia
- How to get a visa
- Flights to Russia
- Russian hotels
- Renting apartments
- Russian currency
- FIFA World Cup 2018
- Submit an article
- Flowers to Russia
- Ask our Expert
Veliky Novgorod city, Russia
The capital city of Novgorod oblast .
Veliky Novgorod - Overview
Veliky Novgorod or Novgorod the Great (just Novgorod until 1999) is a city in the north-west of Russia, the administrative center of Novgorod Oblast. It is one of the oldest and most famous cities in Russia with more than a thousand years of history.
The population of Veliky Novgorod is about 224,800 (2022), the area - 90 sq. km.
The phone code - +7 8162, the postal codes - 173000-173902.
Novgorod city flag
Novgorod city coat of arms.

Novgorod city map, Russia
Novgorod city latest news and posts from our blog:.
7 January, 2022 / Nikolai Bugrov's Summer Dacha in Volodarsk .
27 September, 2020 / The Nikolo-Vyazhischi Convent near Veliky Novgorod .
30 August, 2020 / Staraya Russa - one of the oldest Russian towns .
31 March, 2019 / Vitoslavlitsy Museum of Folk Architecture .
28 January, 2019 / Veliky Novgorod Kremlin .
More posts..
History of Veliky Novgorod
Foundation of novgorod and the novgorod republic.
The official founding year of Novgorod is 859, when it was first mentioned in the chronicle. In 862, the so-called “vocation of the Varangians” led by Rurik took place on Novgorod land, which was the beginning of the formation of the Old Russian state. In 882, after the death of Rurik, Prince Oleg became the ruler of Novgorod. He captured Kiev and moved the capital of the state to it. It was the beginning of the Kievan Rus state.
Novgorod was the second town of the Kievan Rus by cultural, economic, and political influence. In 1045-1050, the Cathedral of St. Sophia was built - the main Orthodox church of Novgorod and one of the oldest preserved stone churches in Russia.
The convenient location of Novgorod at the intersection of trade routes “from the Varangians to the Greeks” made it the largest center of intra-Russian and international trade. Constant contacts of the town with the island of Gotland, German towns and the Hansa led to the opening of the first foreign missions on the territory of Novgorod.
The first attempts of Novgorod to gain independence from the Kievan Rus were taken in the 11th century. Novgorod boyars, with the support of the local population, wanted to get rid of the burden of Kiev taxation and to create their own army. In 1136, due to the retreat of Prince Vsevolod Mstislavovitch from the battlefield at Zhdanaya mountain, he was exiled from Novgorod, and a republican government was established in the region.
From 1136 to 1478, Novgorod was the capital of the Russian medieval state known as the Novgorod Republic. In the 12th century, the Novgorod land included part of the Baltic, part of Karelia, the southern part of Finland, the southern coast of Lake Ladoga, the banks of the Northern Dvina River, and vast areas of the European north up to the Urals.
More Historical Facts…
During the Mongol invasion of Rus, Novgorod avoided destruction due to its remote location. It was the only old Russian town that avoided decline in the 11th-12th centuries. In 1259, with the support of Prince Alexander Nevsky, the Mongols conducted a census in Novgorod to collect tribute. In 1280, in Novgorod, a document was drawn up known as “Russian Truth” - the very first set of laws in Russia. Until the 15th century, Novgorod’s possessions expanded to the east and northeast.
Areas northeast of Novgorod were rich in fur-bearing animals and salt. These resources were of great importance for the economy of the Novgorod Republic, which was based on trade. The town was part of the trade route from Scandinavia to Byzantium. The Russian folk hero Sadko was a merchant from Novgorod.
The Novgorod Republic was characterized by some features of the social system and feudal relations: a significant social and landowning weight of the Novgorod boyars and their active participation in trade and other activities. The main economic factor was not land, but capital. This led to a special social structure of society and an unusual form of government for medieval Rus.
The veche - a gathering of a part of the male population of the town - had broad powers. It invited, judged and expelled Novgorod princes; elected the mayor and the military leader; resolved issues of war and peace; passed and repealed laws; set the size of taxes and duties; elected representatives of the authorities in the Novgorod lands and tried them.
From the 14th century, the Tver and Moscow principalities, and the Grand Duchy of Lithuania attempted to subdue the Novgorod Republic. In 1470, Novgorod people asked the Metropolitan of Kiev to appoint a bishop for them (Kiev belonged to the Grand Duchy of Lithuania at that time). After it, Ivan III, the Grand Prince of Moscow, accused them of treason. In 1471, he announced a military campaign against Novgorod. Moscow troops defeated Novgorod militia during the battle on the Shelon River and took the town.
In 1478, after a series of wars against Moscow, Novgorod lost its independence and the Novgorod Republic ceased to exist. The veche was abolished, the veche bell was taken to Moscow; power in the town was granted to the governors appointed by the Grand Prince of Moscow. A lot of boyar families were expelled from Novgorod.
Novgorod in the 16th-19th centuries
The oprichnina pogrom perpetrated in the winter of 1569/1570 by the army personally headed by Ivan the Terrible inflicted enormous damage on Novgorod. The reason for the pogrom was a denunciation and suspicion of treason. The town was plundered, the property of churches, monasteries and merchants was confiscated, thousands of residents were killed. In 1571, the population of Novgorod was about 5,000 people.
From 1611 to 1617, during the Time of Troubles, Novgorod was occupied by the Swedes. After the occupation, half of the town was burnt down. The population decreased to only about 500 residents. Nikon (the most famous Russian Patriarch) was the metropolitan of Novgorod in 1648-1652.
In 1700, the Great Northern War began. After the defeat at Narva, Peter I hastily prepared the fortifications of Novgorod for a possible siege of the Swedes. Swedish troops did not reach Novgorod; nevertheless, the Novgorod regiment played an important role in the Battle of Poltava in 1709.
In 1703, in connection with the founding of St. Petersburg, the new capital of Russia, a lot of craftsmen from Novgorod were involved in its construction. At the same time, Novgorod finally lost its former importance as a trade center and turned into an ordinary provincial town. In 1727, a separate Novgorod Governorate was formed with its center in Novgorod.
In the first half of the 19th century, Novgorod became the center of military settlements. At the same time, there was almost no industrial production in the town. In 1841-1842, the writer Alexander Herzen was in exile in Novgorod.
One of the brightest pages in the history of Veliky Novgorod in the 19th century was the celebration of the 1000th anniversary of the Russian state in 1862. In honor of this event, a monument to the Millennium of Russia was erected in the center of the Novgorod Kremlin. In 1875, 17,384 people lived in Novgorod, along with military units. 12 small enterprises employed only 63 workers.
Novgorod in the 20th century
Despite the increased interest in its history, Novgorod both at the end of the 19th century and at the beginning of the 20th century remained a typical provincial town of the Russian Empire, despite its status as a regional capital. In 1914, the population of Novgorod was about 28,200 people.
In 1927, as a result of the administrative-territorial reform carried out in the USSR, the Novgorod Governorate became part of Leningrad Oblast. The Leningrad leadership viewed the Novgorod land as a rural region. No industrialization was planned.
From August 15, 1941 to January 20, 1944, during the Second World War, Novgorod was occupied by German and Spanish troops (“The Blue Division”). The war caused huge and in many ways irreparable damage to the monuments of the city itself and its environs. All wooden buildings burned down. The most valuable collections of archeology, history and art were plundered from the Novgorod museum, which was not completely evacuated in time. Almost the entire city infrastructure and industrial enterprises were destroyed, world famous monuments of Novgorod architecture were turned into ruins.
On July 5, 1944, Novgorod Oblast was formed. The transformation of Novgorod into the administrative and economic center of a separate region had a beneficial effect on the acceleration of its restoration. On November 1, 1945, Novgorod was included in the list of 15 Soviet cities subject to priority restoration. In addition, a special decree was issued on the restoration of architectural monuments. One of the first to be restored was the Millennium of Russia monument.
In the post-war years, the presence of large undeveloped areas and wastelands after the dismantling of the rubble of destroyed buildings in the city center made it possible to begin extensive archaeological research. The results of these studies were numerous finds of objects of old Russian art and everyday life. One of the most important finds was the discovery of the first birch bark letter on July 26, 1951. Archaeological research continues to this day.
In the 1950s-1970s, the main restoration work of architectural monuments was carried out. Novgorod became known as the center of all-Union and international tourism. In 1964, not far from the old Yuryev Monastery on the shore of Lake Myachino, the creation of the Vitoslavlitsy Museum of Folk Wooden Architecture began. In 1967, the population of the city was about 107,000 people.
In 1992, 37 unique monuments of old Russian culture in Novgorod were included in the UNESCO World Cultural Heritage List. The population of the city reached its maximum and amounted to 235 thousand people.
On June 11, 1999, the President of the Russian Federation Boris Yeltsin signed the federal law “On renaming the city of Novgorod, the administrative center of Novgorod Oblast, into the city of Veliky Novgorod.” Also in the 1990s, a lot of streets in the city center received their historical names back.
Monuments of Veliky Novgorod

Victory Monument in Veliky Novgorod
Author: Sergey Duhanin

Light tank T-70 in Veliky Novgorod
Author: Konstantin Matekhin

Monument to the Millennium of Russia in Veliky Novgorod
Veliky Novgorod - Features
Veliky Novgorod is deservedly called “the father of Russian cities”. In this place the Russian statehood was born. The city stands on the banks of the Volkhov River, 6 km from Lake Ilmen, 575 km north-west of Moscow and 190 km south-east of St. Petersburg. Due to similar names, Veliky Novgorod is often confused with Nizhny Novgorod. And this applies not only to foreigners, but also to residents of Russia.
The Volkhov River divides the city into two parts: Sofia and Torgovaya (Trade) sides. The Sofia side got its name from the Sofia Cathedral, which is about one thousand years old. The main part of the city is located here, including the Novgorod Kremlin and the historic center, as well as new districts.
In the old days, there was a large market on the Trade side - Torg. Today, it is built up mainly with private houses. The Yaroslav’s Courtyard is also located here. It is a historical and architectural complex on the site where the residence of Yaroslav the Wise (Prince of Novgorod in 988-1015) used to be.
The climate of Veliky Novgorod is moderately continental, with cold snowy winters and moderately warm summers. The average temperature in January is minus 9.2 degrees Celsius, in July - plus 17.3 degrees Celsius.
Novgorod Oblast has a unique transport and geographical position. The main highway, rail, air, and water transport routes connecting St. Petersburg and Moscow pass through its territory. Public transport in the city is represented by buses, trolleybuses.
The region’s largest employer is the chemical fertilizer plant “Acron”, which produces ammonia, saltpeter, nitric acid, and other substances. This enterprise is one of the world’s largest fertilizer producers. The plant in Veliky Novgorod employs about 5 thousand people.
Tourism is gradually becoming more and more important in the city’s economy. Veliky Novgorod is rightly called the city-museum of Old Rus. No other city in Russia has so many remarkable architectural monuments and monumental paintings of the 11th-17th centuries. The architecture of Veliky Novgorod is unique because a lot of old churches have survived here. A significant part of them were erected in the period from the 11th to the 16th centuries. In some churches, old wall paintings have been preserved, which are of great cultural value.
The main discovery made on the territory of Veliky Novgorod is more than 1,000 preserved birch bark letters of various contents written in the 11th-15th centuries. These include business letters, love letters, recipe notes, Bible commentaries, commercial calculations, and even student scribbles.
Main Attractions of Veliky Novgorod
Novgorod Detinets (Kremlin) - the fortress of Veliky Novgorod located on the left bank of the Volkhov River, the oldest preserved kremlin in Russia and the most northern one. The first mention of it in chronicles dates back to 1044. It is an architectural monument of federal significance. Novgorod Detinets as part of the historic center of Veliky Novgorod is included in the UNESCO World Heritage List.
The construction of the stone fortress was completed by the end of the 15th century. About 1.5 kilometers of fortress walls, nine towers, and old churches have survived to this day. A road going through the Novgorod Kremlin leads to the bridge connecting the Sofia and Torgovaya sides of the city. The 41-meter watchtower known as “Kokuy” offers a great view of the entire city and its surroundings.
Today, it is the cultural and tourist center of Veliky Novgorod. Here you can find the main expositions of the Novgorod Museum-Reserve (the exposition of the Old Russian arts and crafts and jewelry in the Chamber of Facets; “History of the Novgorod Region”, “Old Russian icon painting”, “Russian art of the 18th-20th centuries”), restoration workshops, a library, philharmonic society, college of arts, and a music school. The Novgorod Kremlin is surrounded by a spacious park. A walk from the railway station to the Novgorod Kremlin takes about 20 minutes.
Saint Sophia Cathedral (1045-1050) - the main Orthodox church in Veliky Novgorod located on the territory of the Novgorod Kremlin, one of the oldest churches in Russia. Churches of this type were built in Rus only in the 11th century. Sophia Cathedral was built in the Byzantine style, has a pyramidal structure and 6 domes. This is one of the symbols of Veliky Novgorod.
The belfry of St. Sophia Cathedral rises above the Novgorod Kremlin in the form of a wall with five spans in the upper part. This type of structure was invented during the reign of the Novgorod Archbishop Euthymius II and then repeated in Russia only twice.
Monument to the Millennium of Russia (1862) - a magnificent monument more than 15 meters high erected opposite the St. Sophia Cathedral in honor of the millennium anniversary of the legendary vocation of the Varangians to Rus. The monument consists of 128 figures. At the very top you can see an angel - the personification of Orthodoxy and a woman depicting Rus, below - princes, church hierarchs and enlighteners, who took part in important historical events that happened over the thousand-year history of Russia.
Yaroslav’s Courtyard and Torg - an architectural complex located opposite the Novgorod Kremlin, on the other bank of the Volkhov River. Both banks are connected by a pedestrian bridge. Several monuments of the 12th-16th centuries have survived on its territory, including the Nikolsky Cathedral (1113) and the Church of Paraskeva Pyatnitsa (the 13th century). The place was named after Prince Yaroslav the Wise. In old times, fairs were held here. The most recent construction is the arcade of the Gostiny Dvor, which consists of several dozen white-stone arches.
St. George’s (Yuriev) Monastery . Founded in 1030, it is one of the oldest monasteries in Russia, a cultural heritage site of federal significance. The monastery is located on the southern outskirts of Veliky Novgorod on the bank of the Volkhov River. On the territory of the monastery there are St. George, Spassky, and Holy Cross Cathedrals, a four-tiered bell tower 52 m high, and several old churches. Crowned with three silvery domes, St. George’s Cathedral is a wonderful example of Old Russian architecture. The Vitoslavlitsy Museum is located nearby. Yur’yevskoye Highway, 10.
Museum of Folk Wooden Architecture “Vitoslavlitsy” . This open-air exhibition was opened in 1964. 22 wooden architectural monuments of the 16th-20th centuries - churches, residential buildings, and outbuildings - were brought here from different districts of the Novgorod region.
Inside the peasant huts, you can see recreated old interiors: “Winter Life”, “Wedding”, “Wool Felting Craft”, and others. Folk festivals are also held on the territory of the museum. On the second floor of the souvenir shop there is the Museum of Irons with a unique collection of irons of the 18th-20th centuries. Yur’yevskoye Highway, 14.
Center for Musical Antiquities named after V.I. Povetkin . The unique collection of this museum acquaints visitors with the old musical instruments of Rus. Here you can also hear old Russian music. Most of the exhibits date back to the 10th-15th centuries. For the guests of the center, folk songs of the Russian North-West and North are performed. Choir concerts are held on holidays. Il’ina Street, 9b.
Art Museum . The permanent exhibition of this museum is dedicated to Russian art of the 17th-20th centuries. It was opened in the historic building of the Noble Assembly in 2001. The rich collection of the museum includes paintings, drawings, sculptures, miniatures, etc.
Among the most valuable exhibits are paintings created by such masters as K. Bryullov, I. Repin, A. Aivazovsky and sculptures, including M. Antokolsky and M. Vrubel. The museum’s collection of Russian art objects is considered one of the best outside Moscow and St. Petersburg. Sofiyskaya Square, 2.
Museum of Artistic Culture of the Novgorod Land . This museum was opened in one of the buildings of the Desyatinnyy monastery in 2002. The exposition is composed of works by Novgorod artists of the late 20th - early 21st centuries. In addition to paintings, visitors can look at porcelain and glass products made by local craftsmen. Desyatinnyy Lane, 6.
Novgorod city of Russia photos
Pictures of veliky novgorod.

Veliky Novgorod architecture
Author: Elena Abramova

Veliky Novgorod Detinets (Kremlin)
Author: Ismail Soytekinoglu

Veliky Novgorod Fortress
Picturesque churches of Veliky Novgorod

St. Sophia Cathedral in Veliky Novgorod
Author: Zaritsky Igor

Peter and Paul Church in Veliky Novgorod

Church of Clement, Pope of Rome in Veliky Novgorod
Old churches of Veliky Novgorod

Church of the Transfiguration on Ilyin in Veliky Novgorod
Author: Sergey Popov

Myrrhbearers Church in Veliky Novgorod
The questions of our visitors
There are several ways of going from the airport of St. Petersburg to Veliky Novgorod.
The fastest one and of course the most expensive is to go by taxi from the airport straight to the destination city. The price is about 4,500-5,000 Rubles (about 70-80 USD).
Another way is to go by train. From the airport you should go to Vitebsky railway station, departure: 7:53, arrival: 13:06 local time. Also you can go to Moskovsky railway station. There are two trains: the first one departs at 8:12 and the second one - at 17:18). Not sure about the price of the tickets but much cheaper than going by taxi.
The third variant is to go by bus. You should go to the bus station at Obvodnoy Canal Embankment. The first bus departs at 7:30; the latest - at 21:30.
- Currently 3.10/5
Rating: 3.1 /5 (195 votes cast)

IMAGES
VIDEO
COMMENTS
Caprinae Travel was founded by the great bond of warm friendship and experience. Our greatest mission is to gain your trust in our company's introduce America's enthusiastic and adventurous traveler to the land of "Anatolia", the cradle of human civilization, the home of people of various religions in peace, where the heartland of world ...
Caprinae Safaris came on the scene only in early 2006 but its principals are well-known to international big game hunting world, especially in North American continent. Although the company has a short history both Mehmet Alkan and Riza Gozluk have been in the business much longer than fifteen hunting season as they worked for another reputable ...
Caprinae also offers touristic tours in Turkey under the name of Caprinae Travel and provides the guests with VIP quality service. One can visit www.caprinaetravel.com and learn more about these VIP level touristic tours.
Caprinae Safaris offers you the unique opportunity to experience a luxury vacation with unforgettable memories on a 24-43 meter long sailing ship. You enjoy the days on cruise along beautiful bays along the Turkish Riviera. ... An officially licensed, English-speaking interpreter and travel guide is at your service 24 hours, and will be happy ...
Slovakia 1 Species View All Destinations Home Destinations Sightseeing Bow Hunting Testimonials Contac us Monday : 09:00 - 17:00 Thusday : 09:00 - 17:00 Wednesday : 09:00 - 17:00 Thursday : 09:00 - 17:00 Friday : 09:00 - 17:00 Saturday : Closed Sunday : Closed Address : M. Kasapoglu cd. 24/5, 07160 Antalya-TURKEY Phone : +90 242 322 00 27 Email ...
The Caprinae Specialist Group of the International Union for the Conservation of Nature is a network of volunteer experts working on the the ecology, behavior, taxonomy, conservation and management of wild Caprinae (wild sheep, goats, goats-antelopes, muskox and allies) from around the world. We aim to promote the conservation of Caprinae and their habitat, promote research on all aspects of ...
Wild caprinae, including sheep and goats, are an extremely valuable group of mammals. While most live in mountains, some inhabit desert grasslands, tropical forests or even arctic tundra. They range in size from the 30kg goral to the 350kg musk ox and display a variety of horn shapes and sizes as well as coat and body coloration. They are highly prized by hunters on account of their horns and ...
Prominent members include sheep and goats, with some other members referred to as goat antelopes. Some earlier taxonomies considered Caprinae a separate family called Capridae (with the members being caprids), but now it is usually considered either a subfamily within the Bovidae, or a tribe within the subfamily Antilopinae of the family Bovidae, with caprines being a type of bovid.
Worldwide, Caprinae populations receive much attention given their coveted trophy hunts and iconic symbolism for wild and remote places, yet many Caprinae populations are at risk of extinction 1.
Caprinae Travel was founded by the great bond of warm friendship and experience. Our greatest mission is to gain your trust in our company's introduce America's enthusiastic and adventurous traveler to the land of "Anatolia", the cradle of human civilization, the home of people of various religions in peace, where the heartland of world ...
Marc & Cheryl Hansen My wife and I had the pleasure of taking a week tour in Turkey with Caprinae Safaris earlier this year. The folks at Caprinae listened to our desires and skillfully crafted a tour that fit our interests and schedule. This was our first trip to Turkey and it exceeded our expectations; inspiring landscapes, a wealth of interesting antiquities, and a safe, clean, friendly ...
Veliky Novgorod (Russian: Великий Новгород, lit. 'Great Newtown', IPA: [vʲɪˈlʲikʲɪj ˈnovɡərət]), [ 10 ] also known simply as Novgorod (Новгород), is the largest city and administrative centre of Novgorod Oblast, Russia. It is one of the oldest cities in Russia, [ 11 ] being first mentioned in the 9th century.
After spending the last 30-years in the hunting industry, both as a Professional and a client, I can say that Riza and Mehmet, of Caprinae Travel, are world class outfitters, expiditers, booking agents, hunters.
Around 180 kilometers south of St. Petersburg we can find Veliky Novgorod, the oldest city in Russia, whose historical center is declared as a World Heritage Site by UNESCO and it's quite worth a visit. I'll explain you how to get there and what to see.
The Caprinae is one of the most successful bovid subfamilies, with 35 currently recognized species found in mountainous regions across Europe, Asia, Africa, and North America. Their success is due to a generalized form with specializations for montane habitats, a combination which confers flexibility within the alpine biome. The Caprinae tend to be medium-sized ungulates, with a compact form ...
Travel agency Caprinae Travel at Antalya, Muratpaşa, Yeşilbahçe Mah., Metin Kasapoğlu Cad., 24, ☎️ show phone numbers. panoramas. Get directions in Yandex Maps.
552327. Working with others to conserve, protect and enhance fish, wildlife, plants and their habitats for the continuing benefit of the American people. Careers & Internships. Contracting.
Cappadocia, located in Central Anatolia, was named after Cappodocians who settled there about 700 B.C., when craters and chimneys dominated the landscapes. Then million years ago, mount Erciyes erupted violently and Mount Hasan covered the ground with tufa, a soft Stone made from lava, ash and mud. Then the rain, wind and flood waters from the Kızılırmak River shaped the tufa into the most ...
Travel smarter with Capital One at every step of the journey, including booking flights, hotels and rental cars. Get more from your next journey with Capital One Travel.
It is possible to find them at any elevation between 5000 to 11,000 feet (1,500-3,300m). DURATION of the HUNT: Our Bezoar ibex hunting organisations are for 9 days including the 2 days of traveling time. So actual hunting time is 7 days although majority of our hunts ends within the first 4 days. HUNTING METHOD: As each area has its own ...
Sign in to get trip updates and message other travelers.. Veliky Novgorod ; Hotels ; Things to Do ; Restaurants ; Flights ; Vacation Rentals
Veliky Novgorod - Overview Veliky Novgorod or Novgorod the Great (just Novgorod until 1999) is a city in the north-west of Russia, the administrative center of Novgorod Oblast. It is one of the oldest and most famous cities in Russia with more than a thousand years of history.
Caprinae Travel of Turkey recommends you to experience A World of Wonder, A World of Beauty. We want you to EXPLORE ISTANBUL & ENJOY AN UNFORGETTABLE TRIP. CAPRINAE TRAVEL Treasures of Istanbul 5 days M. Kasapoğlu cd.24/5 07160 Antalya- TURKEY Tel: +90 242 322 00 27 Fax: +90 242 322 37 70 Web: www.caprinaetravel.com E-mail: info@caprinaetravel ...
Commercial vehicle travel through units of the National Park Service is generally prohibited by 36 CFR 5.6. Due to the proximity of Zion National Park to a major interstate highway, the growth and development of ... Domesticated Caprinae (sheep and goats) are prohibited from entering the Park, not withstanding 36 CFR definition of a pet (a dog ...