
- Report problem with article
- View revision history

Citation, DOI, disclosures and article data
At the time the article was created Craig Hacking had no recorded disclosures.
At the time the article was last revised Craig Hacking had the following disclosures:
- Philips Australia, Paid speaker at Philips Spectral CT events (ongoing)
These were assessed during peer review and were determined to not be relevant to the changes that were made.
- Diaphragm fluoroscopy
The fluoroscopic sniff test , also known as diaphragm fluoroscopy , is a quick and easy real time fluoroscopic assessment of diaphragmatic motor function (excursion). It is used most often to confirm absence of muscular contraction of the diaphragm during inspiration in patients with phrenic nerve palsy or breathing difficulties following stroke . Chest radiograph demonstrating a newly elevated hemidiaphragm often precedes a sniff test.
In critically unwell patients who can not attend the fluoroscopy unit in the radiology department, bedside US assessment can be used to demonstrate appropriate diaphragmatic movement with normal respiration and when asked to sniff (see case 5).
The following technique is suggested:
ask the patient to practice sniffing before the study
with the patient either standing (preferred) or supine, perform frontal fluoroscopy of the diaphragm at rest, breathing quietly through an open mouth
ask the patient to take a few quick short breaths in with a closed mouth ('sniffs') causing rapid inspiration
occasionally, repeating (3) in the lateral projection is required to evaluate the posterior hemidiaphragms
In normal diaphragmatic motion:
the diaphragm contracts during inspiration: moves downwards
the diaphragm relaxes during expiration: moves upwards
both hemidiaphragms move together
in healthy patients 1-2.5 cm of excursion is normal in quiet breathing 2
3.6-9.2 cm of excursion is normal in deep breathing 2
up to 9 cm can be seen in young or athletic individuals in deep inspiration 2
excursion in women is slightly less than men 2
In abnormal diaphragmatic motion:
the affected hemidiaphragm does not move downwards during inspiration
paradoxical motion can occur
Interpretation
Absence of diaphragmatic movement confirms phrenic nerve palsy in the appropriate clinical setting. A mass anywhere along the course of the phrenic nerve requires further workup, usually with neck and chest CT. A hilar mass due to lung cancer is the most common finding on CT and a classic exam case.
Normal diaphragmatic excursion can also be impaired in patients with:
previous diaphragmatic trauma or surgery
neuromuscular disorders
previous stroke
- 1. Nason LK, Walker CM, McNeeley MF et-al. Imaging of the diaphragm: anatomy and function. Radiographics. 2012;32 (2): E51-70. doi:10.1148/rg.322115127 - Pubmed citation
- 2. Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135 (2): 391-400. doi:10.1378/chest.08-1541 - Pubmed citation
- Nason L, Walker C, McNeeley M, Burivong W, Fligner C, Godwin J. Imaging of the Diaphragm: Anatomy and Function. RadioGraphics. 2012;32(2):E51-70. doi:10.1148/rg.322115127 - Pubmed
Incoming Links
- Diaphragmatic paralysis
- Phrenic nerve palsy
- Ultrasound diaphragmatic sniff test
- Left hilar mass causing phrenic nerve palsy
- Large right diaphragmatic hernia
- Hemidiaphragmatic paralysis
- Abnormal sniff test
- Normal sniff test
- Phrenic nerve palsy with positive sniff test
Promoted articles (advertising)
ADVERTISEMENT: Supporters see fewer/no ads
By Section:
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers


- Introduction
- Palp/Percus
- Auscultation
Palpation/Percussion
Thoracic expansion:.
- Is used to evaluate the symmetry and extent of thoracic movement during inspiration.
- Is usually symmetrical and is at least 2.5 centimeters between full expiration and full inspiration.
- Can be symmetrically diminished in ankylosing spondylitis .
- Can be unilaterally diminished in chronic fibrotic lung disease , extensive lobar pneumonia, large pleural effusions, bronchial obstruction and other disease states.
Percussion:
Percussion is the act of tapping on a surface, thereby setting the underlying structures in motion, creating a sound and palpable vibration. Percussion is used to determine whether underlying structures are fluid-filled, gas-filled, or solid. Percussion:
- Penetrates 5 - 6 centimeters into the chest cavity.
- May be impeded by a very thick chest wall.
- Produces a low-pitched, resonant note of high amplitude over normal gas-filled lungs.
- Produces a dull, short note whenever fluid or solid tissue replaces air filled lung (for example lobar pneumonia or mass) or when there is fluid in the pleural space (for example serous fluid, blood or pus).
- Produces a hyperresonant note over hyperinflated lungs (e.g. COPD ).
- Produces a tympanitic note over no lung tissue (e.g. pneumothorax ).
Diaphragmatic excursion:
- Can be evaluated via percussion.
- Is 4-6 centimeters between full inspiration and full expiration.
- May be abnormal with hyperinflation , atelectasis , the presence of a pleural effusion , diaphragmatic paralysis, or at times with intra-abdominal pathology.
- Open access
- Published: 28 February 2019
A narrative review of diaphragm ultrasound to predict weaning from mechanical ventilation: where are we and where are we heading?
- Peter Turton ORCID: orcid.org/0000-0001-7974-3031 1 , 2 ,
- Sondus ALAidarous 1 , 3 &
- Ingeborg Welters 1 , 2
The Ultrasound Journal volume 11 , Article number: 2 ( 2019 ) Cite this article
17k Accesses
29 Citations
53 Altmetric
Metrics details
The use of ultrasound to visualize the diaphragm is well established. Over the last 15 years, certain indices of diaphragm function, namely diaphragm thickness, thickening fraction and excursion have been established for mechanically ventilated patients to track changes in diaphragm size and function over time, to assess and diagnose diaphragmatic dysfunction, and to evaluate if these indices can predict successful liberation from mechanical ventilation. In the last 2 years, three meta-analyses and a systematic review have assessed the usability of diaphragmatic ultrasound to predict successful weaning. Since then, further data have been published on the topic.
Conclusions
The aim of this narrative review is to briefly describe the common methods of diaphragmatic function assessment using ultrasound techniques, before summarizing the major points raised by the recent reviews. A narrative summary of the most recent data will be presented, before concluding with a brief discussion of future research directions in this field.
There has been much interest in the use of diaphragm ultrasound as a tool of measuring and tracking atrophy, in particular to identify patients who will wean from mechanical ventilation, and who will remain free of ventilatory support afterwards. Two meta-analyses and a systematic review have been published on the topic in the last 2 years, and more work is being produced. The aim of this narrative review is briefly re-iterate what is being measured with diaphragm ultrasound, to summarize the most recent findings from these reviews and meta-analyses, and to provide an update of current work produced after these reviews.
The diaphragm in critical care: what do we know?
The effects of atrophy of the diaphragm secondary to mechanical ventilation have been recently described; Goligher found that the development of diaphragm atrophy was associated with prolonged duration of mechanical ventilation, increased ICU length of stay, and a higher rate of complications [ 1 ]. Interestingly, patients who showed an increase in diaphragmatic thickness during their critical illness were also at higher risk of prolonged mechanical ventilation, with excessive respiratory effort as a possible underlying trigger. The authors did acknowledge that tissue oedema from fluid resuscitation may also contribute to this thickening. Diaphragmatic thickness has been shown to reduce by 6% [ 2 ] or 7.5% [ 3 ] per day in mechanically ventilated patients. However, a further study demonstrated that although nearly half of the patients in their study did suffer atrophy, the same proportion experienced no loss, and a further 10% actually had increases in diaphragmatic thickness [ 4 ]. A recent study in mechanically ventilated children suggested that diaphragmatic atrophy occurs at an average rate of 3.4% per day and is worsened by the use of neuromuscular blockade [ 5 ]. However, two papers failed to demonstrate diaphragmatic atrophy using ultrasound [ 6 , 7 ]. However, one of these studies was in extubated survivors of sepsis (82% of which had either severe sepsis or septic shock) who were approached after a period of at least 5 days of mechanical ventilation, compared to controls. However, the authors concede that these results were based on a single measurement at a point in the patients’ recovery from sepsis rather than during the acute episode.
Ultrasound and the diaphragm
Visualization of the diaphragm with ultrasound has been possible for well over 40 years [ 8 ]. However, only recently diaphragmatic ultrasound has been used to assess diaphragm function and size during mechanical ventilation. There are two commonly used measurements derived from ultrasound: diaphragm excursion and diaphragm thickness [ 9 ]. Diaphragm excursion is usually measured using a phased array probe, with the probe positioned in the subcostal margin in the mid-clavicular line, with the aim of imaging the posterior third of the diaphragm (Fig. 1 ). Although some studies have used B-mode imaging to determine diaphragmatic excursion [ 10 ], the use of M-mode produces images that visualize the movement of the diaphragm over time and allows accurate measurement of diaphragmatic displacement over a respiratory cycle (Figs. 2 and 3 ) [ 11 ]. In healthy volunteers, diaphragmatic excursion is known to vary with sex and height and can be reliably performed in a recumbent or supine position [ 12 ]. Excursion is known to positively correlate with lung inspiratory volumes [ 13 , 14 ], and is higher during forced inspiratory breathing [ 10 ].
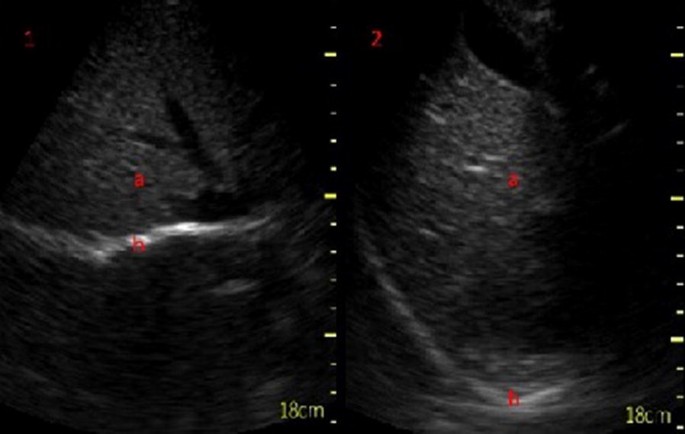
Subcostal view of the diaphragm (b) in B-mode at end inspiration (1) and at expiration (2) seen below the liver (a)
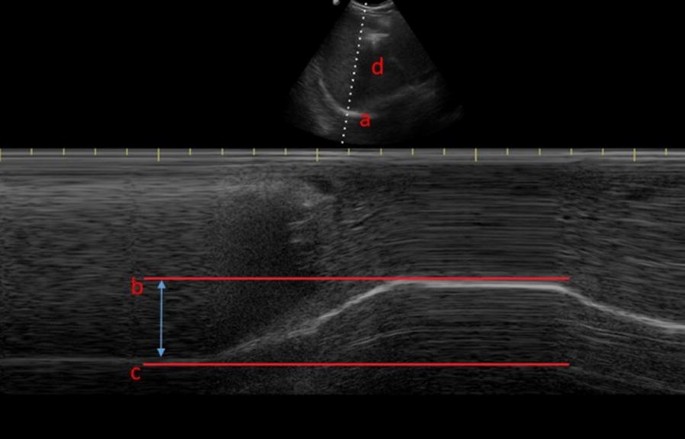
Diaphragm excursion as assessed via M-mode ultrasonography, where a is the diaphragm, b is at the end of a deep inspiratory effort, c is at end expiration and d is the liver

M-mode ultrasonography demonstrating three tidal inspiratory efforts ( a ) and a deep inspiratory effort ( b )
Diaphragm thickness is measured in the zone of apposition, using a higher-frequency (> 10 MHz) linear probe, to view the diaphragm as a three-layered structure, sandwiched between the two echogenic layers of the pleura and the peritoneum (Fig. 4 ) [ 15 ]. Both B- and M-mode techniques can be used to measure thickness [ 16 ]. Diaphragm thickness has been previously correlated with the strength of the diaphragm [ 17 ], but not the endurance or fatigability [ 18 ]. It appears to be thicker in an upright position, compared to supine posture [ 19 ], can be measured at expiration or end inspiration, and in both tidal and maximal breathing. Comparing expiratory with inspiratory thickness gives the thickening fraction, which is usually denoted as [(End Inspiratory Thickness − End Expiratory Thickness)/End Expiratory Thickness] [ 20 ] and is an indicator of the work of breathing [ 21 ]. These measurements can be used to form a definition of diaphragm dysfunction, although there is variation in this definition: It has been defined as a thickening fraction of less than 20% or a tidal excursion of less than 10 mm [ 22 ], based on the presence of paradoxical movement in the case of the paralyzed diaphragm [ 9 ], or using non-ultrasound methods such as measurement of twitch pressures [ 23 ]. Regardless, ultrasound techniques have been shown to outperform traditional techniques such as fluoroscopy in diagnosing diaphragm dysfunction [ 24 ].
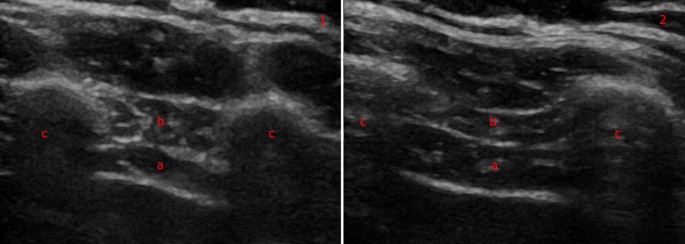
Diaphragm thickness in B-mode thoracic view at end expiration (1) and inspiration (2) in a heathy volunteer. The diaphragm can be seen between two echogenic layers (a) with the intercostal compartment above (b). The two muscle layers sit between two ribs ( c )
Summary of current literature
In 2017, a systematic review [ 25 ] and a meta-analysis [ 26 ] have been performed, assessing the evidence on diaphragm ultrasound and its ability to predict successful weaning from mechanical ventilation. Two further meta-analyses have been published in 2018 [ 27 , 28 ], and together these reviews assessed the combined work of more than 30 individual papers (excluding further 3 papers that looked exclusively at lung rather than diaphragm ultrasound).
The systematic review [ 25 ] focused on the use of diaphragmatic ultrasound in four key areas: to diagnose diaphragmatic dysfunction, to predict successful weaning from mechanical ventilation, to determine if ultrasound can assess muscular workload against other known measurements such as transdiaphragmatic pressure [ 29 ], and to describe variations in diaphragm atrophy across studies.
With respect to weaning from mechanical ventilation, four studies were analyzed, two of which described diaphragm excursion either by M-mode ultrasound [ 30 ] or by measuring organ displacement [ 31 ]. The two remaining studies assessed diaphragmatic thickening fraction [ 32 , 33 ]. All four studies concluded that their respective measurements can predict successful extubation or weaning failure, with cut-off values of 11–14 mm in excursion and 30–36% in thickening fraction being most sensitive and specific.
The three meta-analyses are broadly similar in their aims and results. A possible reason may lie in the slight differences to the selection criteria; Li et al. reviewed only publications in English and defined weaning failure as the requirement for re-intubation within 48 h, whereas Llamas-Álverez et al. included publications in all languages and had a much broader definition of weaning failure to include death, unscheduled non-invasive ventilation, tracheostomy formation or the failure of a spontaneous breathing trial within 72 h, and Qian defined weaning failure more broadly as a failed spontaneous breathing trial, re-intubation, the use of non-invasive ventilation, or death. Li and Llamas-Álverez found similar AUC characteristics for the use of diaphragm thickening fraction (0.83 versus 0.87). Llamas-Álverez et al. concluded that thickening fraction may help to predict weaning failure, and Li et al. concluded that the either measurement is suitable to predict successful extubation. Qian found that pooled specificity for predicting weaning success was similar to the work of Llamas-Álverez, and also found that weaning failure was higher in the presence of diaphragmatic dysfunction, and that both excursion and thickening fraction were higher in patients who were successfully weaned.
All papers acknowledge the heterogeneity in the studies analyzed. The definition of weaning failure varied amongst individual studies, with re-intubation limits set at either 48 or 72 h, and some studies included the use of non-invasive ventilation to define weaning failure. Inclusion criteria are different for each of the studies; while some studies recruited patients during their first spontaneous breathing trial [ 32 ], another study included only patients who had already had a failed trial [ 33 ]. Further differences arise from the ultrasonic technique chosen; in Li’s meta-analysis, 4 of the studies were conducted with the patient in a supine position, while patients were semi-recumbent in the remaining 9 studies. Although probe position was consistent in all studies, probe frequency and ultrasound machine manufacturer varied considerably, with 12 different types of ultrasound machines being used, covering a range of frequencies from 3.5 to 10 MHz. There is also variation in the patient populations, particularly with respect to age and sex. It is known that age negatively correlates with excursion in deep breathing, and that females have less diaphragmatic excursion [ 12 ]. Many of the studies included both left and right sides of the diaphragm, but some reported measurements of the right side only, probably because there is greater difficulty in imaging the left diaphragm due to the lung obscuring the view [ 13 ]. Finally, there is variation regarding the time point during a spontaneous breathing trial at which measurements are taken, with ultrasound images being obtained at the start or end of spontaneous breathing; some investigators assessed diaphragmatic function after extubation, others during mechanically ventilation with further variation in the ventilatory mode used. It has been suggested that pre-extubation is the best time to perform diaphragm ultrasound to assess the diaphragm at a time point when it may be fatigued. A protocol for a new study performing ultrasounds at regular intervals throughout 2 h of spontaneous breathing has been published, but the results are not yet available [ 34 ].
Where are we now?
Since the publication of the systematic reviews, several studies have reported diaphragmatic ultrasound parameters as a means of predicting extubation success. Our search strategy used MEDLINE only; we searched for papers published after 1st January 2017 until to the current date of writing (August 2018). Using the search string (Ultraso* AND Diaphagm* AND (critical* OR intensive OR sepsis OR mechanical * OR ventilat*) yielded 89 results. After having excluded papers that already appeared in the four systematic reviews and papers covering different topics as assessed on title and/or abstract), we identified 18 new papers since 2017 that have not been part of a systematic review.
Newer studies
Newly identified studies can be divided into three categories. In the first, there are studies in which diaphragm thickening or excursion are used alone to predict successful weaning. In the second, diaphragm ultrasound is compared to another technique; and in the third, these techniques are combined to see if they increase predictive accuracy.
A recent study describes cut-offs for diaphragm thickening to predict successful weaning prior to a spontaneous breathing trial [ 35 ]. Its results are in keeping with previously established cut-offs for patients weaning from pressure support [ 36 ]. However, there are also conflicting results. For example, a study evaluating diaphragm excursion using spleen and liver displacement found that displacement of the organs by 1.2 cm was the best cut-off for predicting successful extubation [ 37 ]. However, poor agreement between solid organ movement and diaphragm excursion has been described before [ 38 ]. Another recent study found that diaphragmatic excursion, and not thickening fraction, was the best predictor of extubation failure in patients undergoing their first spontaneous breathing trial [ 39 ]. The most recent reliability study has established values of inter- (0.987) and intra-observer variability (0.986) that are within the higher range of Intra Class Correlation (ICC) coefficients established in the systematic review [ 40 ].
Newer studies—comparative approaches
Dres and colleagues compared the performance of diaphragm ultrasound against tracheal pressure measurements obtained during supra-maximal phrenic nerve stimulation during a spontaneous breathing trial [ 23 ]. They not only found that a lower stimulated pressure than previously accepted was associated with optimum sensitivity and specificity for liberation from mechanical ventilation [ 41 ], but also described that a thickening fraction of greater than 25.8% gave equivalent accuracy of prediction in comparison to phrenic nerve stimulation, with AUC–ROC values of 0.80 and 0.82 for phrenic nerve stimulation and diaphragm thickening fraction, respectively.
Newer studies—combined approaches
Combining diaphragmatic ultrasound with echocardiography may be a promising route for prediction of successful weaning, particularly in view of potential cardiac causes for a failed respiratory wean [ 42 ]. The ratio of mitral Doppler inflow velocity (E) to annular tissue Doppler wave velocity (Ea, E /Ea ratio) has been measured with transthoracic echocardiography alongside diaphragmatic excursion in patients who were extubated after a successful spontaneous breathing trial (SBT) [ 43 ]. The authors found that respiratory failure within 48 h of extubation could be predicted from both E /Ea and left ventricular ejection fraction values, but that reintubation within a week of extubation was more accurately predicted by diaphragmatic excursion.
Another study combined echocardiography with lung ultrasound and assessment of diaphragmatic excursion to assess if all three combined could predict extubation failure in patients undergoing a trial of pressure support ventilation [ 44 ]. The results were confirmed in a smaller sub-study of patients breathing via a T-tube, although out of the three modalities, diaphragm ultrasound contributed least predicting successful weaning. Furthermore, a recent small observational study has combined echocardiography and lung ultrasound for assessment of aeration with diaphragmatic ultrasound, and reported that lung aeration and markers of diastolic dysfunction were the only strong predictors of successful extubation [ 45 ].
Another combined approach combined diaphragm thickening fraction with the Rapid Shallow Breathing Index (RSBI). First described in 1991 [ 46 ], RSBI is defined as the ratio of the respiratory frequency to the tidal volume [ 47 ], with a cut-off value of 100–105 breaths/min/liter being associated with successful extubation [ 46 , 48 ]. A recent study found that RSBI alone, in comparison to measurements derived from diaphragm ultrasound, was most accurate in predicting success of extubation, with an ROC–AUC of 0.96 and a sensitivity and specificity of 100% [ 49 ]. This supports earlier work that the sensitivity, specificity and positive predictive value of a thickening fraction cut-off 36% were comparable to RSBI, but ultimately lower than it [ 33 ]. However, the combination of RSBI with diaphragm thickening fraction of greater than 26% was a more accurate predictor of successful weaning from mechanical ventilation than RSBI alone [ 50 ]. The authors concluded that thickening fraction of the right diaphragm alone was as accurate as this combined approach, and suggested that thickening fraction could replace RSBI as the most commonly used weaning parameter in the future.
Future directions
As ultrasound technology progresses, it may be possible for clinicians to estimate diaphragm thickness and excursion using portable, hand-held devices. A recent study used both linear and phased arrays probes of a hand-held ultrasound device to assess diaphragmatic thickness and excursion, respectively, compared to a standard ultrasound device [ 22 ]. Good agreement was noted between the two devices, with ICCs of greater than 0.9 noted in all indices of measurement except for maximal excursion. Based on a definition of diaphragmatic dysfunction as tidal excursion of less than 10 mm, the detection of dysfunction was comparable between the two devices, and good inter-rater reliability was also seen. Stronger agreement between the two devices was seen in the measurement of diaphragm thickness compared to measurement of excursion, possibly because of the hand-held device lacking an M-mode for accurate measurement of excursion.
A third measurement, the contraction velocity, has also been evaluated recently. Contraction velocity is an extension of diaphragm excursion, dividing excursion by the time to reach maximal excursion [ 9 ]. None of the systematic reviews assessed contraction velocity in the prediction of successful weaning. A study of elderly ventilated patients found that right-sided contraction velocity had a similar AUC as right-sided excursion (labeled in the study as diaphragmatic motion), and that both of these were more predictive for successful weaning from mechanical ventilation than RSBI [ 51 ]. Contraction velocity has been shown to have high sensitivity and specificity, and only performed slightly worse than RSBI in a study of younger patients [ 49 ]. A more recent study, however, found that there was no difference in contraction velocity between patients who were successfully extubated, compared to those who were re-intubated [ 52 ]. It is not clear why there is such variation in results, and further research on conduction velocity is required, along with standardization whether velocity is measured over tidal or maximal inspiratory efforts. The same authors found that multiplying the diaphragmatic excursion ( E ) by the inspiratory time ( I ) to give a diaphragmatic excursion-time index gave values that were significantly higher in patients who had been successfully extubated compared to those who failed extubation [ 53 ]. The differences were still significant regardless as to whether the measurements were performed during spontaneous breathing or after extubation. However, significance was lost during pressure-assist ventilation modes.
Speckle tracking can detect tissue motion and distortion [ 54 ]. In healthy volunteers, it has been used to successfully assess diaphragmatic strain under pressure support ventilation [ 55 ] and was weakly but significantly associated with caudal diaphragm displacement [ 56 ]. This technique may provide useful information about the diaphragm during controlled mechanical ventilation, but as yet, there are no studies examining speckle tracking in the critical care population. Similarly, an “area method” [ 57 ] assessing diaphragm motion in two dimensions, correlates with lung volume using both B and M-mode ultrasound in healthy volunteers, and can be performed on both sides of the chest.
Further research focuses on the prediction of successful extubation in particular patient groups. For example, a recent study demonstrated that diaphragm thickness measured before anesthetic induction correlates with time to extubation in patients undergoing liver transplants. Time to extubation after the procedure was higher in patients with pre-operative end expiratory diaphragm thickness of less than 2 mm [ 58 ].
In patients with Chronic Obstructive Pulmonary Disease (COPD), diaphragmatic ultrasound may predict successful weaning on one side, but could also serve to predict success of non-invasive ventilation. In this context, it has been reported that COPD patients with diaphragm dysfunction (diagnosed by a thickening fraction of less than 20%), who require non-invasive ventilation, were 4.4 times more likely to need intubation, more often proceeded to tracheostomies, and had increased length of stay in ICU and hospital mortality [ 59 ]. These results were in line with an earlier smaller study that also described an association of diaphragm dysfunction with Non-Invasive Ventilation (NIV) failure and increased mortality [ 60 ]. However, reduced diaphragmatic thickening itself is not a risk factor for acute exacerbation of COPD [ 61 ].
Diaphragmatic ultrasound has been extensively studied as a predictor of successful weaning from mechanical ventilation, and continues to be studied. It remains difficult to draw general conclusions from individual studies due to the marked variation in study design and population. Even, definitions such as a failed breathing trial or failed extubation have not been standardized across studies, rendering comparison between outcome measures impossible. As yet, defined cut-offs for measurements of diaphragmatic ultrasound have been agreed, and there are no randomized control trials available. Although diaphragmatic ultrasound is a promising diagnostic tool, greater standardization of protocols, outcome measures and ventilatory settings is required for further research and clinical application.
Abbreviations
area under the curve
area under the curve (receiver-operator characteristic)
Chronic Obstructive Pulmonary Disease
intra-class correlation coefficient
intensive care unit
non-invasive ventilation
spontaneous breathing trial
Rapid Shallow Breathing Index
Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS et al (2018) Mechanical ventilation–induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med 197:204–213
Article CAS Google Scholar
Grosu HB, Lee YI, Lee J, Eden E, Eikermann M, Rose KM (2012) Diaphragm muscle thinning in patients who are mechanically ventilated. Chest 142:1455–1460
Article Google Scholar
Zambon M, Beccaria P, Matsuno J (2016) Mechanical ventilation and diaphragmatic atrophy in critically ill patients: an ultrasound study. Crit Care Med 43:29–38
Google Scholar
Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brade D et al (2015) Evolution of diaphragm thickness during mechanical ventilation. impact of inspiratory effort. Am J Respir Crit Care Med 192:1080–1088
Glau CL, Conlon TW, Himebauch AS, Yehya N, Weiss SL, Berg RA et al (2018) Progressive diaphragm atrophy in pediatric acute respiratory failure. Pediatr Crit Care Med 19:406–411
Cartwright MS, Kwayisi G, Griffin LP, Sarwal A, Walker FO, Harris JM et al (2013) Quantitative neuromuscular ultrasound in the intensive care unit. Muscle Nerve 47:255–259
Baldwin CE, Bersten AD (2014) Alterations in respiratory and limb muscle strength and size in patients with sepsis who are mechanically ventilated. Phys Ther 94:68–82
Doust BD, Baum JK, Maklad NF, Doust VL (1975) Ultrasonic evaluation of pleural opacities. Radiology 114:135–140
Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F et al (2013) Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med 39:801–810
Testa A, Soldati G, Giannuzzi R, Berardi S, Portale G, Gentilone Silveri N et al (2011) Ultrasound M-mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med Biol 37:44–52
Houston JG, Morris AD, Howie CA, Reid JL (1992) Technical report: quantitative assessment of diaphragmatic movement-A reproducible method using ultrasound quantitative assessment of diaphragmatic movement a reproducible method using ultrasound. Clin Radiol 46:405–407
Scarlata S, Mancini D, Laudisio A, Benigni A, Antonellia Incalzi R (2018) Reproducibility and clinical correlates of supine diaphragmatic motion measured by M-mode ultrasonography in healthy volunteers. Respiration 96:259–266
Boussuges A, Gole Y, Blanc P (2009) Diaphragmatic motion studied by M-mode ultrasonography: methods, reproducibility, and normal values. Chest 135:391–400
Houston JG, Angus RM, Cowan MD, McMillan NC, Thomson NC, Houston M et al (1994) Ultrasound assessment of normal hemidiaphragmatic movement: relation to inspiratory volume. Thorax 49:500–503
Haji K, Royse A, Green C, Botha J, Canty D, Royse C (2016) Interpreting diaphragmatic movement with bedside imaging, review article. J Crit Care 34:56–65
Boon AJ, Harper CJ, Ghahfarokhi LS, Strommen JA, Watson JC, Sorenson EJ (2013) Two-dimensional ultrasound imaging of the diaphragm: quantitative values in normal subjects. Muscle Nerve 47:884–889
Cardenas LZ, Santana PV, Caruso P, Ribeiro de Carvalho CR, Pereira de Albuquerque AL (2018) Diaphragmatic ultrasound correlates with inspiratory muscle strength and pulmonary function in healthy subjects. Ultrasound Med Biol 44:786–793
Holtzhausen S, Unger M, Lupton-Smith A, Hanekom S (2018) An investigation into the use of ultrasound as a surrogate measure of diaphragm function. Heart Lung 47:418–424
Hellyer NJ, Andreas NM, Bernstetter AS, Cieslak KR, Donahue GF, Steiner EA et al (2017) Comparison of diaphragm thickness measurements among postures via ultrasound imaging. PM&R 9:21–25
Vivier E, Roche-Campo F, Brochard L, Mekontso Dessap A (2017) Determinants of diaphragm thickening fraction during mechanical ventilation: an ancillary study of a randomised trial. Eur Respir J 50:1700783
Vivier E, Dessap AM, Dimassi S, Vargas F, Lyazidid A, Thille AW et al (2012) Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med 38:796–803
Gursel G, Inci K, Alasgarova Z (2018) Can diaphragm dysfunction be reliably evaluated with pocket-sized ultrasound devices in intensive care Unit? Crit Care Res Pract. Article ID: 5192647
Dres M, Goligher EC, Dubé B-P, Morawiec E, Dangers L, Reuter D et al (2018) Diaphragm function and weaning from mechanical ventilation: an ultrasound and phrenic nerve stimulation clinical study. Ann Intensive Care 8:53
Houston JG, Fleet M, Cowan MD, McMillan NC (1995) Comparison of ultrasound with fluoroscopy in the assessment of suspected hemidiaphragmatic movement abnormality. Clin Radiol 50:95–98
Zambon M, Greco M, Bocchino S, Cabrini L, Beccaria PF, Zangrillo A (2017) Assessment of diaphragmatic dysfunction in the critically ill patient with ultrasound: a systematic review. Intensive Care Med 43:29–38
Llamas-Álvarez AM, Tenza-Lozano EM, Latour-Pérez J (2017) Diaphragm and lung ultrasound to predict weaning outcome. Chest 152:1140–1150
Li C, Li X, Han H, Cui H, Wang G, Wang Z (2018) Diaphragmatic ultrasonography for predicting ventilator weaning: a meta-analysis. Medicine (Baltimore) 97:e10968
Qian Z, Yang M, Li L, Chen Y (2018) Ultrasound assessment of diaphragmatic dysfunction as a predictor of weaning outcome from mechanical ventilation: a systematic review and meta-analysis. BMJ Open 8:21189
Goligher EC, Laghi F, Detsky ME, Farias P, Murray A, Brace D et al (2015) Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity. Intensive Care Med 41:642–649
Kim Y, Hee HJ, Suh J, Hong RSB, Koh Y, Lim CM (2011) Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med 39:2627–2630
Jiang JR, Tsai TH, Jerng JS, Yu CJ, Wu HD, Yang PC (2004) Ultrasonographic evaluation of liver/spleen movements and extubation outcome. Chest 126:179–185
Dinino E, Gartman EJ, Sethi JM, Mccool D (2014) Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 69:423–427
Ferrari G, De Filippi G, Fabrizio E, Panero F, Volpicelli G, Apra F (2014) Diaphragm ultrasound as a new index of discontinuation from mechanical ventilation. Crit Ultrasound J 6:8
Zhou P, Zhang Z, Hong Y, Cai H, Zhao H, Xu P et al (2017) The predictive value of serial changes in diaphragm function during the spontaneous breathing trial for weaning outcome: a study protocol. BMJ Open 7:e015043
Samanta S, Singh RK, Baronia AK, Poddar B, Azim A, Gurjar M (2017) Diaphragm thickening fraction to predict weaning—a prospective exploratory study. J Intensive Care 5:62
Blumhof S, Wheeler D, Thomas K, Mccool F, Mora J (2016) Change in diaphragmatic thickness during the respiratory cycle predicts extubation success at various levels of pressure support ventilation. Lung 194:519–525
Hayat A, Khan A, Khalil A, Asghar A (2017) Diaphragmatic excursion: does it predict successful weaning from mechanical ventilation? J Coll Physicians Surg Pak 27:743–746
PubMed Google Scholar
Haji K, Royse A, Tharmaraj D, Haji D, Botha A, Royse C (2015) Diaphragmatic regional displacement assessed by ultrasound and correlated to subphrenic organ movement in the critically ill patients—an observational study. J Crit Care 30:439.e7–439.e13
Yoo J-W, Lee SJ, Lee JD, Kim HC (2018) Comparison of clinical utility between diaphragm excursion and thickening change using ultrasonography to predict extubation success. Korean J Intern Med 33:331–339
Dhungana A, Khilnani G, Hadda V, Guleria R (2017) Reproducibility of diaphragm thickness measurements by ultrasonography in patients on mechanical ventilation. World J Crit Care Med 6:185–189
Dres M, Dubé B-P, Mayaux J, Delemazure J, Reuted D, Brochard L et al (2017) Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med 195:57–66
Chien JY, Lin MS, Huang YCT, Chien YF, Yu CJ, Yang PC (2008) Changes in B-type natriuretic peptide improve weaning outcome predicted by spontaneous breathing trial. Crit Care Med 36:1421–1426
Luo L, Li Y, Chen X, Sun B, Li W, Gu W et al (2017) Different effects of cardiac and diaphragm function assessed by ultrasound on extubation outcomes in difficult-to-wean patients: a cohort study. BMC Pulm Med 17:161
Silva S, Ait Aissa D, Cocquet P, Hoarau L, Ruiz J, Ferre F et al (2017) Combined thoracic ultrasound assessment during a successful weaning trial predicts postextubation distress. Anesthesiology 127:666–674
Haji K, Haji D, Canty DJ, Royse AG, Green C, Royse CF (2018) The impact of heart, lung and diaphragmatic ultrasound on prediction of failed extubation from mechanical ventilation in critically ill patients: a prospective observational pilot study. Crit Ultrasound J 10:13
Yang KL, Tobin MJ, Presberg KW (1991) A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Eng J Med 324:1445–1450
Karthika M, Al Enezi FA, Pillai LV, Arabi YM (2016) Rapid shallow breathing index. Ann Thorac Med 11:167–176
Epstein SK (1995) Etiology of extubation failure and the predictive value of the rapid shallow breathing index. Am J Respir Crit Care Med 152:545–549
Banerjee A, Mehrotra G (2018) Comparison of lung ultrasound-based weaning indices with rapid shallow breathing index: are they helpful? Indian J Crit Care Med 22:435–440
Pirompanich P, Romsaiyut S (2018) Use of diaphragm thickening fraction combined with rapid shallow breathing index for predicting success of weaning from mechanical ventilator in medical patients. J intensive care 6:6
Huang D, Ma H, Zhong W, Wang X, Wu Y, Qin T et al (2017) Using M-mode ultrasonography to assess diaphragm dysfunction and predict the success of mechanical ventilation weaning in elderly patients. J Thorac Dis 9:3177–3186
Palkar A, Mayo P, Singh K, Koenig S, Narasimhan M, Singh A et al (2018) Serial diaphragm ultrasonography to predict successful discontinuation of mechanical ventilation. Lung 196:363–368
Palkar A, Narasimhan M, Greenberg H, Singh K, Koenig S, Mayo P et al (2018) Diaphragm excursion-time index: a new parameter using ultrasonography to predict extubation outcome. Chest 153:1213–1220
Haaksma M, Roel Tuinman P, Heunks L (2017) Ultrasound to assess diaphragmatic function in the critically ill-a critical perspective. Ann Transl Med 5:114
Hatam N, Goetzenich A, Rossaint R et al (2014) A novel application for assessing diaphragmatic function by ultrasonic deformation analysis in noninvasively ventilated healthy young adults. Ultraschall der Medizin Eur J Ultrasound 35:540–546
Orde SR, Boon AJ, Firth DG, Vilarrage HR, Sekiguchi H (2015) Diaphragm assessment by two dimensional speckle tracking imaging in normal subjects. BMC Anesthesiol 16:43
Skaarup SH, Løkke A, Laursen CB (2018) The Area method: a new method for ultrasound assessment of diaphragmatic movement. Crit Ultrasound J 10:15
Sharma A, Karna ST, Tandon M, Pandey CK, Chaturvedi R, Vyas V et al (2018) Use of ultrasound-guided preoperative diaphragmatic thickness as a predictor of postoperative weaning failure in recipients and donors scheduled for living donor liver transplant surgery. Saudi J Anaesth 12:406–411
Marchioni A, Castaniere I, Tonelli R, Fantini R, Fontana M, Tabbi L et al (2018) Ultrasound-assessed diaphragmatic impairment is a predictor of outcomes in patients with acute exacerbation of chronic obstructive pulmonary disease undergoing noninvasive ventilation. Crit Care 22:109
Antenora F, Fantini R, Iattoni A, Castaniere I, Sdanganelli A, Livrieri F et al (2017) Prevalence and outcomes of diaphragmatic dysfunction assessed by ultrasound technology during acute exacerbation of COPD: a pilot study. Respirology 22:338–344
Eryüksel E, Cimşit C, Bekir M, Cimsit C, Karakurt S (2017) diaphragmatic thickness fraction in subjects at high-risk for COPD exacerbations. Respir Care 62:1565–1570
Download references
Authors’ contributions
PT wrote and devised the manuscript. SA provided the ultrasound images for the figures and assisted in the literature search. IW assisted in the editing and writing of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Competing interests.
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Ethical approval and consent to participate, publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and affiliations.
Critical Care Unit, Royal Liverpool University Hospital, Liverpool, UK
Peter Turton, Sondus ALAidarous & Ingeborg Welters
Institute of Aging and Chronic Disease, University of Liverpool, Liverpool, UK
Peter Turton & Ingeborg Welters
Institute of Infection and Global Health, University of Liverpool, Liverpool, UK
Sondus ALAidarous
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Peter Turton .
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Reprints and permissions
About this article
Cite this article.
Turton, P., ALAidarous, S. & Welters, I. A narrative review of diaphragm ultrasound to predict weaning from mechanical ventilation: where are we and where are we heading?. Ultrasound J 11 , 2 (2019). https://doi.org/10.1186/s13089-019-0117-8
Download citation
Received : 21 December 2018
Accepted : 08 February 2019
Published : 28 February 2019
DOI : https://doi.org/10.1186/s13089-019-0117-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Muscle atrophy
- Diaphragm ultrasound
- Thickening fraction
- Correspondence
- Open access
- Published: 15 June 2023
“Under pressure”: should we use diaphragm excursion to predict weaning success in patients receiving pressure support ventilation?
- Emma Sabourin 1 ,
- Christophe Carpentier 1 ,
- Christopher Lai 1 , 2 ,
- Xavier Monnet 1 , 2 &
- Tài Pham 1 , 3
Critical Care volume 27 , Article number: 238 ( 2023 ) Cite this article
1656 Accesses
3 Citations
Metrics details
The Original Article was published on 14 January 2023
We read with great interest the recent article published in Critical Care by Huan Ma et al. entitled “Using automatic speckle tracking imaging to measure diaphragm excursion and predict the outcome of mechanical ventilation weaning” [ 1 ]. Diaphragm ultrasound is an interesting technique to better understand weaning physiology and outcomes, and although we agree with the authors' perspective, we think that the results of the study should be interpreted with caution.
This prospective, multicenter, observational study aimed to evaluate the ability of diaphragm excursion (assessed with automatic speckle tracking) to predict weaning outcome. The authors found a significant correlation between the automatic measurement of mean excursion and velocity in speckle tracking imaging and its manual measurement. After analyzing the receiver operating characteristic (ROC) curve, they showed that diaphragmatic velocity and mean excursion were promising high diagnostic values for prolonged weaning. Yet, the diagnostic value of diaphragm excursion was moderate for predicting in-hospital death, withdrawal of treatment and weaning failure.
We would like to raise a few points that appear important when using ultrasound to evaluate diaphragm function and that might impact the interpretation of the study.
First of all, diaphragm excursion was measured in patients receiving invasive mechanical ventilation in pressure support mode with support set at 10–12 cm H 2 O. It appears to us that the value of assessing diaphragm excursion under assisted mechanical ventilation, such as in this study, should be subject to caution and cannot be interpreted as the patient’s own respiratory muscle strength.
Indeed, as well demonstrated in several studies [ 2 , 3 , 4 ], it is not possible to differentiate which part of the diaphragmatic excursion measured is due to the external force applied by the ventilator (passive), and which part of the excursion is due to the diaphragmatic contraction (active). Measures of diaphragm excursion under assisted mechanical ventilation will consequently be overestimated because the patient’s diaphragmatic contraction is added to the passive excursion generated by the ventilator in pressure support mode.
Conceptually, this would not allow for weaning outcome prediction, and in their study, Zombon et al. [ 2 ] emphasized the fact that diaphragm excursion measures should be limited to patients with spontaneous breathing, as it is not a marker per se of diaphragm contraction or respiratory effort but a marker of diaphragm movement is highly dependent on inspired volumes.
M. Llamas-Álvarez and co-workers [ 3 ] also highlighted the fact that diaphragm excursion is only relevant in patients without ventilator support, and showed that diaphragm excursion interpretation entails several biases as diaphragm excursion may vary depending on several parameters such as the patient’s positioning, and thoracic or abdominal pressure variation. These authors even concluded that diaphragm excursion should not be used to assess diaphragm function.
Using diaphragm excursion to predict weaning success should therefore be measured in patients undergoing a spontaneous breathing trial, such as a T-piece trial (disconnecting the patient from the ventilator) or a ZEEP trial (decreasing the pressure support to minimal values with PEEP set at 0 cm H 2 0). In this situation, the excursion measured will hence apprehend the diaphragmatic contraction without the impact of the pressure support generated by the ventilator.
Diaphragm thickening fraction, which is also measured with ultrasound, is another interesting technique as several studies demonstrated its reliability to predict extubation success [ 4 ]. It might even be superior to diaphragm excursion in this indication: two studies have demonstrated a significant correlation between the diaphragmatic tidal thickening fraction and the diaphragmatic pressure–time product in patients receiving noninvasive ventilation after extubation and in healthy subjects and intubated patients with pressure support ventilation [ 4 ].
Moreover, some studies have shown an interesting and feasible method for predicting weaning success using the measurement of the right diaphragm thickening fraction in combination with the rapid shallow breathing index (RSBI). This combination has been shown to improve the precision of successful weaning prediction when compared with RSBI alone [ 5 ].
Therefore, the prediction of weaning success in patients undergoing assisted breathing trials should be evaluated by diaphragmatic thickening fraction as it is less impacted by pressure support variation [ 2 ].
In a nutshell, we think diaphragm excursion measurement is an interesting approach but should be done in patients with spontaneous breathing without pressure support to be able to predict weaning success. Nonetheless, this study has shown promising results regarding the feasibility and reliability of speckle tracking imaging, with high correlation values. Further research on diaphragm function assessment to predict weaning outcome is needed.
Availability of data and materials
Not applicable.
Huang D, Song F, Luo B, Wang S, Qin T, Lin Z, et al. Using automatic speckle tracking imaging to measure diaphragm excursion and predict the outcome of mechanical ventilation weaning. Crit Care. 2023;27:18.
Article PubMed PubMed Central Google Scholar
Zambon M, Greco M, Bocchino S, Cabrini L, Beccaria PF, Zangrillo A. Assessment of diaphragmatic dysfunction in the critically ill patient with ultrasound: a systematic review. Intensive Care Med. 2017;43:29–38.
Article PubMed Google Scholar
Llamas-Álvarez AM, Tenza-Lozano EM, Latour-Pérez J. Diaphragm and lung ultrasound to predict weaning outcome: systematic review and meta-analysis. Chest. 2017;152:1140–50.
Sferrazza Papa GF, Pellegrino GM, Di Marco F, Imeri G, Brochard L, Goligher E, et al. A review of the ultrasound assessment of diaphragmatic function in clinical practice. Respiration. 2016;91:403–11.
Pirompanich P, Romsaiyut S. Use of diaphragm thickening fraction combined with rapid shallow breathing index for predicting success of weaning from mechanical ventilator in medical patients. J Intensive Care. 2018;6:6.
Download references
Author information
Authors and affiliations.
Service de Médecine Intensive-Réanimation, Hôpital de Bicêtre, DMU CORREVE, FHU SEPSIS, Groupe de Recherche CARMAS, Hôpitaux Universitaires Paris-Saclay, AP-HP, 94270, Le Kremlin-Bicêtre, France
Emma Sabourin, Christophe Carpentier, Christopher Lai, Xavier Monnet & Tài Pham
INSERM UMR S_999, Pulmonary Hypertension: Pathophysiology and Novel Therapies, University Paris-Saclay, Hôpital Marie Lannelongue, Le Plessis-Robinson, France
Christopher Lai & Xavier Monnet
INSERM U1018, Equipe d’Epidémiologie Respiratoire Intégrative, CESP, Université Paris-Saclay (UVSQ)—Université Paris-Sud, 94807, Villejuif, France
You can also search for this author in PubMed Google Scholar
Contributions
ES, CC and TP provided concept and design. ES, CC and TP performed drafting of the manuscript. ES, CC, CL, XM and TP performed critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Tài Pham .
Ethics declarations
Ethical approval and consent to participate, competing interests.
C.L. received honoraria for lectures from Sedana Medical. XM is a member of the medical advisory board of Pulsion Medical Systems. He received lecture fees from Pulsion Medical Systems and Baxter Healthcare. E.S., C.C and T.P have no competing interests to declare.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sabourin, E., Carpentier, C., Lai, C. et al. “Under pressure”: should we use diaphragm excursion to predict weaning success in patients receiving pressure support ventilation?. Crit Care 27 , 238 (2023). https://doi.org/10.1186/s13054-023-04504-8
Download citation
Received : 16 May 2023
Accepted : 23 May 2023
Published : 15 June 2023
DOI : https://doi.org/10.1186/s13054-023-04504-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Critical Care
ISSN: 1364-8535
- Submission enquiries: [email protected]
- Open access
- Published: 22 October 2021
Diaphragmatic excursion is correlated with the improvement in exercise tolerance after pulmonary rehabilitation in patients with chronic obstructive pulmonary disease
- Masashi Shiraishi ORCID: orcid.org/0000-0001-5410-1331 1 , 2 ,
- Yuji Higashimoto 1 ,
- Ryuji Sugiya 1 ,
- Hiroki Mizusawa 1 ,
- Yu Takeda 1 ,
- Shuhei Fujita 1 ,
- Osamu Nishiyama 2 ,
- Shintarou Kudo 3 ,
- Tamotsu Kimura 1 ,
- Yasutaka Chiba 4 ,
- Kanji Fukuda 1 ,
- Yuji Tohda 2 &
- Hisako Matsumoto 2
Respiratory Research volume 22 , Article number: 271 ( 2021 ) Cite this article
4567 Accesses
8 Citations
4 Altmetric
Metrics details
In patients with chronic obstructive pulmonary disease (COPD), the maximum level of diaphragm excursion (DE max ) is correlated with dynamic lung hyperinflation and exercise tolerance. This study aimed to elucidate the utility of DE max to predict the improvement in exercise tolerance after pulmonary rehabilitation (PR) in patients with COPD.
This was a prospective cohort study. Of the 62 patients with stable COPD who participated in the outpatient PR programme from April 2018 to February 2021, 50 completed the programme. Six-minute walk distance (6MWD) was performed to evaluate exercise tolerance, and ultrasonography was performed to measure DE max . Responders to PR in exercise capacity were defined as patients who demonstrated an increase of > 30 m in 6MWD. The receiver operating characteristic (ROC) curve was used to determine the cut-off point of DE max to predict responses to PR.
Baseline levels of forced expiratory volume in 1 s, 6MWD, maximum inspiratory pressure, DE max and quadriceps muscle strength were significantly higher, and peak dyspnoea of modified Borg (mBorg) scale score was lower in responders (n = 30) than in non-responders (n = 20) to PR (p < 0.01). In multivariate analysis, DE max was significantly correlated with an increase of > 30 m in 6MWD. The area under the ROC curve of DE max to predict responders was 0.915, with a sensitivity and specificity of 83% and 95%, respectively, at a cut-off value of 44.9 mm of DE max .
DE max could adequately predict the improvement in exercise tolerance after PR in patients with COPD.
Chronic obstructive pulmonary disease (COPD) is a progressive disease characterised by minimally reversible airflow limitation [ 1 ]. The main feature of COPD is the inability of patients to cope with their activities of daily life due to shortness of breath. Although the pathophysiological mechanisms involved in the development of dyspnoea and poor exercise tolerance in patients with COPD are complex, dynamic lung hyperinflation (DLH) plays a central role [ 2 ] by increasing ventilatory workload and decreasing the pressure-generating capacity of the inspiratory muscles.
Pulmonary rehabilitation (PR) is a non-pharmacological intervention and has been reported to improve dyspnoea, exercise capacity and quality of life of patients with COPD [ 3 ]. Owing to a body of evidence, PR is now established as the standard of care for patients with COPD [ 4 ]. However, not all patients with COPD benefit from PR to the same extent. Therefore, identifying patients who are likely to achieve maximum benefit from the PR programme is crucial. So far, several studies have shown that severe airflow limitation or poor exercise tolerance at baseline may predict a better response to PR [ 5 , 6 ], but another study has reported inconsistent findings [ 7 ]. Furthermore, one study reported that patients with severe dyspnoea did not respond well to PR and patients with milder dyspnoea responded well [ 8 ].
Considering the role of DLH in the development of dyspnoea and poor exercise tolerance in patients with COPD, objective measures that reflect the degree of DLH may help in identifying good responders to PR. Previously, we reported that there was an association between increased dyspnoea due to DLH on exercise and decreased exercise capacity in patients with COPD and reduced mobility of the diaphragm, which was assessed by the maximum level of diaphragm excursion (DE max ) using ultrasonography [ 9 ]. Other research groups reported the utility of ultrasonographic assessment of diaphragmatic mobility in COPD in understanding its association with 6-min walk distance (6MWD), dyspnoea [ 10 ] and increased mortality [ 11 ].
However, there have been no reports on the association between diaphragmatic mobility and the effect of PR to improve exercise tolerance. The primary aim of this study is to clarify the role of DE max to predict the improvement in exercise tolerance after PR in patients with COPD.

Materials and methods
Study design and subjects.
This was a single-centre, observational, prospective cohort study. The study included 62 patients with clinically stable COPD who visited the Department of Respiratory Medicine and Allergology, Kindai University Hospital, between April 2018 and February 2021. The exclusion criteria included unstable medical conditions that could cause or contribute to breathlessness, such as metabolic, cardiovascular or other respiratory diseases, or any other disorders that could interfere with exercise testing, such as neuromuscular diseases or musculoskeletal problems. This study was approved by the Ethics Committee of Kindai University School of Medicine. Written informed consent was obtained from all participants.
Measurements
All participants underwent ultrasonography (Xario 200, Toshiba, Tokyo, Japan) for the assessment of their DE max . Using the liver as an acoustic window (Fig. 1 A), a convex 3.5 MHz probe was used to measure the excursions of the right hemidiaphragm according to the techniques mentioned in previous studies [ 9 , 12 , 13 ]. The M-mode cursor was rotated and placed on the axis of diaphragmatic displacement on the stored image, and displacement measurements were performed. Measurements were performed during each of the three deep breaths, and DE max was measured (Fig. 1 B). The maximum value obtained for the three deep breaths was used. 6MWD was performed to evaluate walking capacity according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) statement [ 14 , 15 , 16 ]. All participants performed the 6MWD test before and after the PR programme, and the magnitude of their perceived breathlessness and their leg fatigue was rated using a 1–10-point Borg scale. Responders to PR in exercise capacity were defined as those who demonstrated more than 30 m increase in 6MWD after the PR programme, which was the definition of minimal clinically important difference (MCID) for 6MWD [ 17 ].
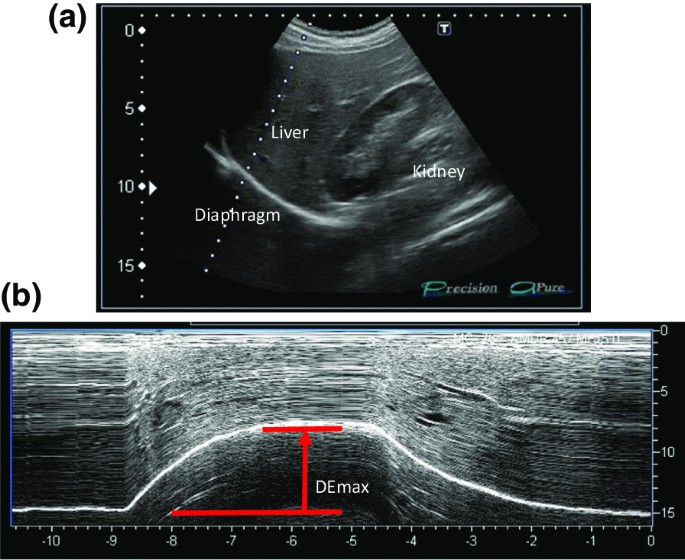
Representative image of the right diaphragm. The probe was positioned below the right costal margin between the midclavicular and anterior axillary lines. A Two-dimensional ultrasonographic image of the right hemidiaphragm (B-mode). Diaphragmatic movements were recorded in M-mode during deep breathing (DE max ) ( B )
Spirometry (CHESTAC-800, Chest, Tokyo, Japan) was performed following the 2005 ATS/ERS recommendations [ 18 ] for measuring forced vital capacity (FVC), forced expiratory volume in 1 s (FEV 1 ) and inspiratory capacity. Respiratory muscle strength was assessed by measuring the maximum inspiratory pressure (PI max ) generated against an occluded airway at residual volume [ 19 ] (SP-370, Fukuda Denshi, Tokyo, Japan). A hand-held dynamometer (μTasF-1, Anima Corp., Tokyo) was used to measure quadriceps muscle strength (QMS). The impact of COPD on health status was assessed using the COPD assessment test (CAT), a patient-completed questionnaire on eight items, namely, cough, phlegm, chest tightness, breathlessness, limited activities, confidence leaving home, sleeplessness and energy. The scores for each of the items range from 0 to 5 points, resulting in a CAT total score ranging from 0 to 40 points [ 20 ], and MCID of CAT is 2 points [ 21 ]. In all patients with COPD, emphysema was evaluated by computed tomography of the chest. A SYNAPSE VINCENT volume analyser (FUJIFILM Medical, Tokyo, Japan) was used to measure the low attenuation area (%LAA).
Rehabilitation programme
The outpatient PR programme was conducted twice a week for 12 weeks (24 sessions), including aerobic exercise training (ergometer and walking exercise) at 60–70% of peak workload for 20–40 min and upper- and lower-limb muscle strength training for 10–20 min.
Sample size
The sample size was estimated using R software. The analysis based on 6MWD data from the PR programme revealed that 40 subjects were required if the expected area under the curve (AUC) below the receiver operating characteristic (ROC) curve was 0.80, the power was 90%, and the significance level was 0.01. Furthermore, we anticipated a dropout from the PR programme. Thus, we set the sample size to 50 participants.
Statistical analysis
Responders and non-responders were compared using t -test, the Wilcoxon rank-sum test or χ 2 test, as appropriate. The paired t -test or the Wilcoxon signed-rank test was used to evaluate the changes in the parameters before and after the PR programme. The Pearson correlation coefficient was used to analyse the relationship between changes in 6MWD and independent variables because changes in 6MWD were normally distributed. Additionally, multivariate logistic regression models were used to assess the ability of variables to predict a response to PR. The ROC curve method was used to assess the ability of DE max to predict a response to PR. All statistical analyses were performed using the JMP software programme (JMP®, Version 14; SAS Institute Inc., Cary, NC, USA).
Out of the 62 patients included in the study, 50 completed the PR programme (Fig. 2 ). Two patients dropped out because of severe exacerbation of COPD, and 10 patients discontinued the PR owing to the coronavirus pandemic. Table 1 presents the baseline characteristics of the participants. After the PR programme, scores for CAT, 6MWD, peak dyspnoea and leg fatigue of the modified Borg (mBorg) scale, and QMS improved significantly (Table 2 ). Thirty patients showed an increase of > 30 m in 6MWD after PR (responders: 60%), and 20 patients (40%) were defined as non-responders. Baseline levels of %FEV 1 , 6MWD, PI max , DE max and QMS were significantly higher and those of CAT score and peak dyspnoea of mBorg scale were significantly lower in responders than in non-responders (Table 1 ). Changes in 6MWD were significantly correlated with baseline levels of CAT, %FEV 1 , peak dyspnoea of mBorg scale, PI max , DE max (Fig. 3 ) and QMS and marginally correlated with baseline levels of 6MWD (Table 3 ).
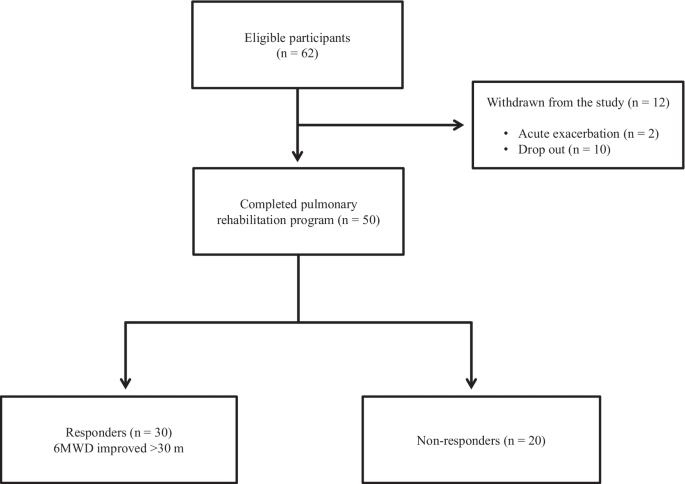
Study flow diagram. COPD chronic obstructive pulmonary disease, PR pulmonary rehabilitation, 6MWD 6-min walk distance
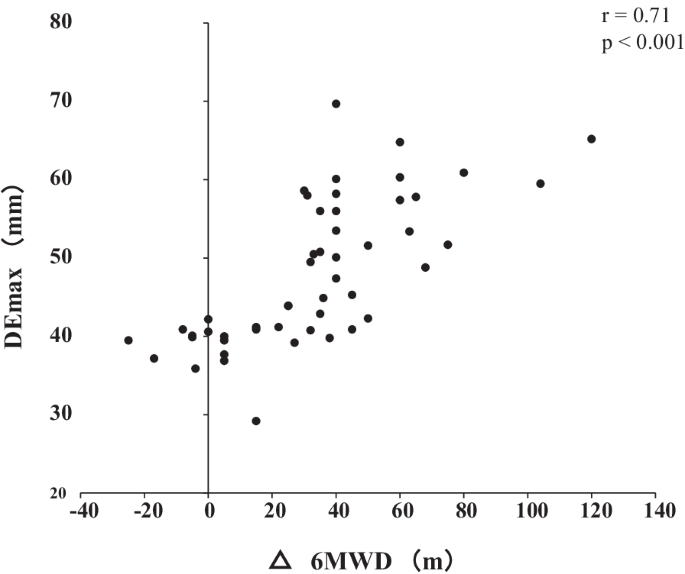
Relationship between DE max and the changes in 6MWD after pulmonary rehabilitation. Changes in 6MWD were significantly positively correlated with DE max (r = 0.72; p < 0.001). DE max maximum diaphragmatic excursion, 6MWD 6-min walk distance
In multivariate analysis, DE max alone significantly contributed to the prediction of responders (Table 4 , Model 1). When using PI max instead of DE max because PI max and DE max showed a strong association (r = 0.73), both PI max and %FEV 1 contributed to the prediction (Table 4 , Model 2). The area under the ROC curve of DE max to predict the responders was 0.915, with a sensitivity of 83% and a specificity of 95% at a cut-off value of 44.9 mm of DE max (Fig. 4 ). The significance of DE max in the predictability of responders remained even when the analysis was confined to severe patients (%FEV 1 < 50%, n = 23; AUC = 0.88, sensitivity = 70% and specificity = 100% at a cut-off value of 44.9 mm).
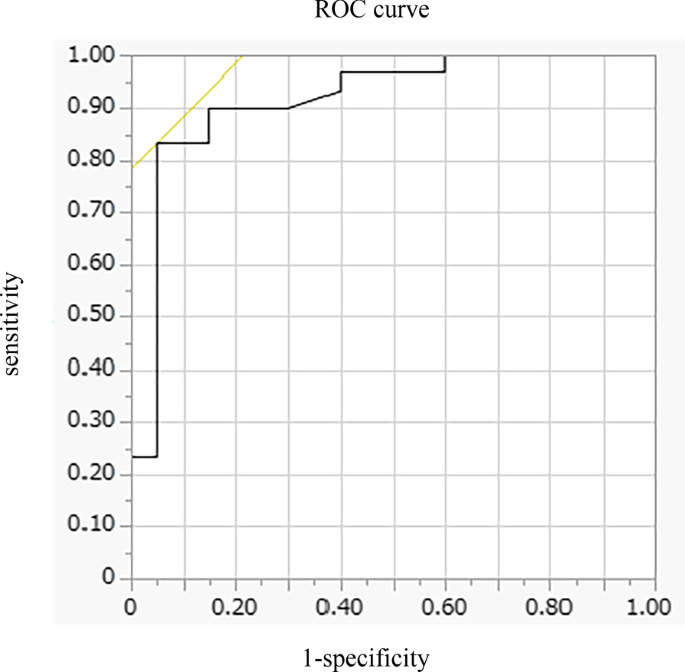
Receiver operating characteristic (ROC) curve for baseline DE max in relation to the response to pulmonary rehabilitation. ROC curve estimates the ability of DE max to predict a clinically important improvement in 6MWD (> 30 m) after pulmonary rehabilitation (AUC = 0.915, sensitivity = 83% and specificity = 95% at a cut-off point of 44.9 mm of DE max ). AUC area under the curve, 6MWD 6-min walk distance, DE max maximum diaphragmatic excursion
This is the first study to demonstrate the utility of DE max to predict the responsiveness of patients with COPD to 12-week PR. In this study, multivariate analysis revealed that greater baseline DE max was the only factor that predicted the responsiveness to PR, independent of baseline %FEV 1 . Additionally, the model using DE max had better prediction performance than that using PI max . The AUC of DE max to predict the 30 m or more improvement in 6MWD after the PR was 0.915, with a sensitivity of 83% and a specificity of 95% at 44.9 mm.
PR is beneficial to patients with chronic respiratory disease, including COPD [ 3 ], and generally improves exercise performance, health-related quality of life and dyspnoea [ 22 ], which was confirmed in this study. Ideally, PR was proven to be effective in all patients, but the response to PR varies considerably between individual patients [ 8 , 23 , 24 , 25 ]. Indeed, in this study, the improvement in 6MWD was less than that in MCID in 40% of the patients regardless of the degree of severity of COPD. Therefore, identifying predictors of a response is crucial in ensuring better PR efficacy and personalisation of PR programmes for patients with COPD.
In this study, the baseline values of %FEV 1 , PI max , DE max , QMS and 6MWD were positively associated with Δ6MWD in univariate analysis, suggesting that a better baseline condition was associated with a higher proportion of patients who achieved MCID after PR. These findings are consistent with those of previous studies that showed that patients with higher levels of %FEV 1 or FEV 1 /VC achieved greater improvement in 6MWD after PR [ 7 , 26 , 27 ] and a study in which patients with milder mMRC scores could achieve MCID of 6MWD after PR [ 8 ], but not for those with worst mMRC score, although others studies showed contradictory results [ 5 , 6 , 28 , 29 , 30 ] or found no significant baseline characteristics to predict a response to PR [ 31 ]. The discrepancy between the findings cannot be fully explained, but it might be due to the differences in the studied population and strength or length of PR. In this study, the mean %FEV 1 of the participants was 56.0%, which was relatively higher than that of other studies (mean %FEV 1 of 40–50% in most studies) [ 5 , 6 , 28 ], despite similar inclusion criteria throughout the studies, i.e., not limited to severe COPD in most studies. Thus, no ceiling effect with a PR programme that included high-intensity load exercise training for 20–40 min was observed in our population.
In this study, an important finding is that greater DE max at baseline was the only factor that predicted the responders in 6MWD after PR. In addition, the model using DE max had better prediction performance than that using PI max . The high predictability of DE max may be because of its strong association with DLH and dyspnoea during exercise, as reported previously [ 9 ]. DLH is involved in the development of dyspnoea, and both are important factors to determine the improvement in 6MWD in patients with COPD. Therefore, DE max that reflects the degree of DLH and dyspnoea during exercise was superior to other physiological indices to predict responders.
Furthermore, the virtuous cycle observed in our PR programme that included high-intensity load exercise training might be a result of the improvement in ventilation pattern. Improving the ventilation pattern would be easier with greater DE max , as shown in studies of mechanically ventilated patients [ 32 ], which may have reduced dyspnoea during exercise after 12 weeks of PR and improved exercise tolerance. Exercise therapy is a central component of PR, which significantly reduces blood lactate levels during exercise, reduces minute ventilation and improves exercise tolerance [ 33 ]. The high-intensity load exercise training, which is performed at 60–80% of the maximum oxygen uptake, has a higher physiological effect than low exercise load. Patients with greater DE max may be able to perform higher load training, which resulted in effective PR.
Diaphragm ultrasonography has been widely and successfully used to identify diaphragmatic dysfunction by showing its association with 6MWD, dyspnoea [ 10 ], extubation failure in mechanically ventilated patients [ 32 ], and increased mortality [ 11 ]. Recently, Lewinska and Shahnazzaryan proposed its use in pulmonary physiotherapy of patients with COPD [ 34 ]. In most previous studies, diaphragm ultrasonography was used to assess DE max , i.e., the measurement of the excursion of the right hemidiaphragm, as used in this study, and diaphragm thickness that assessed the length and thickness of the zone of apposition of the diaphragm against the rib cage [ 35 , 36 ]. However, it is difficult to measure diaphragm thickness in patients with severe COPD because the length of the zone of apposition is shorter in patients with COPD than that in control subjects [ 37 ], whereas it is easy to measure DE max, which shows high intra- and inter-observer reliability [ 38 ]. Bhatt et al. showed that improvement in 6MWD was associated with that in DE max during forced expiration when the effectiveness of pursed lips breathing was assessed in the PR of patients with COPD [ 39 ]. Corbellini et al. demonstrated greater improvement in DE max during inspiration after PR, which was associated with an increase in the inspiratory capacity [ 40 ]. The normal and cut-off values of DE max during normal respiration, forced respiration, and voluntary sniffing have been described for each gender [ 38 ]. Thus, DE max would be a useful and reliable measure for incorporation into the PR assessment. Furthermore, in clinical settings, this objective measure of DE max has additional advantages as it requires minimum effort in patients and can be applied to the PR programme at home if portable ultrasonography is used. However, the assessment of DE max has a limitation. The procedures pertaining to positioning of patients, breathing patterns, and the selected hemidiaphragm are not standardised at present, which may hamper the routine use of DE max at this moment. Standardisation of these parameters would further facilitate the use of DE max in clinical settings and for research purpose.
There are some limitations to this study. This was a single-centre study involving a relatively small number of participants, and their baseline condition might have been relatively preserved. Nonetheless, 46% of the participants showed FEV 1 < 50%, and the utility of DE max was also observed in these patients with severe airflow limitation. Furthermore, in this study, few patients discontinued the PR programme, except for patients who discontinued during the coronavirus pandemic, which indicates that there was no severe mismatch between the PR programme and the patients’ ability to successfully complete this programme. As another limitation, we did not evaluate any malnutrition factors, which could be an important determinant of diaphragmatic mobility. Nonetheless, DE max was a stronger predictor of the effectiveness of PR than other parameters, including QMS or lung function using multivariate analysis. Further studies with a large number of patients are required, and the utility of DE max should be examined in patients with the most severe form of COPD with a low-intensity load exercise programme.
In conclusion, DE max , which is a reliable and easy to perform measurement, could adequately predict the improvement in exercise tolerance after PR in patients with COPD. Assessment of DE max could aid in making medical decisions associated with therapeutic strategies.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Chronic obstructive pulmonary disease
Dynamic lung hyperinflation
- Pulmonary rehabilitation
6-Min walk distance
Minimal clinically important difference
Forced vital capacity
Forced expiratory volume in 1 s
Maximum inspiratory pressure
Quadriceps muscle strength
COPD assessment test
Low attenuation area
Area under the curve
Receiver operating characteristic
Modified Borg
Global initiative for chronic obstructive lung disease (gold). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2020 report. . https://goldcopd.org/gold-reports/ last accessed: 20 Jan 2020.
Gagnon P, Guenette JA, Langer D, Laviolette L, Mainguy V, Maltais F, Ribeiro F, Saey D. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J COPD. 2014;9:187–201.
Google Scholar
Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, Hill K, Holland AE, Lareau SC, Man WD, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-64.
Article PubMed Google Scholar
Dong J, Li Z, Luo L, Xie H. Efficacy of pulmonary rehabilitation in improving the quality of life for patients with chronic obstructive pulmonary disease: evidence based on nineteen randomized controlled trials. Int J Surg. 2020;73:78–86.
Boutou AK, Tanner RJ, Lord VM, Hogg L, Nolan J, Jefford H, Corner EJ, Falzon C, Lee C, Garrod R, et al. An evaluation of factors associated with completion and benefit from pulmonary rehabilitation in COPD. BMJ Open Respir Res. 2014;1:e000051.
Article PubMed PubMed Central Google Scholar
Costi S, Crisafulli E, Trianni L, Beghe B, Faverzani S, Scopelliti G, Chetta A, Clini E. Baseline exercise tolerance and perceived dyspnea to identify the ideal candidate to pulmonary rehabilitation: a risk chart in COPD patients. Int J Chron Obstruct Pulmon Dis. 2019;14:3017–23.
van Ranst D, Otten H, Meijer JW, van’t Hul AJ. Outcome of pulmonary rehabilitation in COPD patients with severely impaired health status. Int J Chron Obstruct Pulmon Dis. 2011;6:647–57.
Garrod R, Marshall J, Barley E, Jones PW. Predictors of success and failure in pulmonary rehabilitation. Eur Respir J. 2006;27:788–94.
Article CAS PubMed Google Scholar
Shiraishi M, Higashimoto Y, Sugiya R, Mizusawa H, Takeda Y, Fujita S, Nishiyama O, Kudo S, Kimura T, Chiba Y, et al. Diaphragmatic excursion correlates with exercise capacity and dynamic hyperinflation in COPD patients. ERJ Open Res 2020, 6.
Paulin E, Yamaguti WPS, Chammas MC, Shibao S, Stelmach R, Cukier A, Carvalho CRF. Influence of diaphragmatic mobility on exercise tolerance and dyspnea in patients with COPD. Respir Med. 2007;101:2113–8.
Yamaguti WPdS, Paulin E, Salge JM, Chammas MC, Cukier A, de Carvalho CRF. Diaphragmatic dysfunction and mortality in patients with COPD. J Bras Pneumol. 2009;35:1174–81.
Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135:391–400.
Testa A, Soldati G, Giannuzzi R, Berardi S, Portale G, Gentiloni Silveri N. Ultrasound M-Mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med Biol. 2011;37:44–52.
Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7.
Article Google Scholar
Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, McCormack MC, Carlin BW, Sciurba FC, Pitta F, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–46.
Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, Hill CJ, Lee AL, Camillo CA, Troosters T, Spruit MA, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1447–78.
Polkey MI, Spruit MA, Edwards LD, Watkins ML, Pinto-Plata V, Vestbo J, Calverley PMA, Tal-Singer R, Agustí A, Bakke PS, et al. Six-minute-walk test in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:382–6.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38.
Lisboa C, Munoz V, Beroiza T, Leiva A, Cruz E. Inspiratory muscle training in chronic airflow limitation: comparison of two different training loads with a threshold device. Eur Respir J. 1994;7:1266–74.
Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–54.
Kon SSC, Canavan JL, Jones SE, Nolan CM, Clark AL, Dickson MJ, Haselden BM, Polkey MI, Man WDC. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med. 2014;2:195–203.
Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006:CD003793.
Spruit MA, Gosselink R, Troosters T, Kasran A, Van Vliet M, Decramer M. Low-grade systemic inflammation and the response to exercise training in patients with advanced COPD. Chest. 2005;128:3183–90.
de Torres JP, Pinto-Plata V, Ingenito E, Bagley P, Gray A, Berger R, Celli B. Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest. 2002;121:1092–8.
Troosters T, Gosselink R, Decramer M. Exercise training in COPD: how to distinguish responders from nonresponders. J Cardiopulm Rehabil. 2001;21:10–7.
Vagaggini B, Costa F, Antonelli S, De Simone C, De Cusatis G, Martino F, Santerini S, Paggiaro P. Clinical predictors of the efficacy of a pulmonary rehabilitation programme in patients with COPD. Respir Med. 2009;103:1224–30.
Scott AS, Baltzan MA, Fox J, Wolkove N. Success in pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Can Respir J. 2010;17:219–23.
Crisafulli E, Gorgone P, Vagaggini B, Pagani M, Rossi G, Costa F, Guarriello V, Paggiaro P, Chetta A, de Blasio F, et al. Efficacy of standard rehabilitation in COPD outpatients with comorbidities. Eur Respir J. 2010;36:1042–8.
Zanini A, Chetta A, Gumiero F, Della Patrona S, Casale S, Zampogna E, Aiello M, Spanevello A. Six-minute walking distance improvement after pulmonary rehabilitation is associated with baseline lung function in complex COPD patients: a retrospective study. Biomed Res Int. 2013;2013:1–6.
Ragaselvi S, Janmeja AK, Aggarwal D, Sidana A, Sood P. Predictors of response to pulmonary rehabilitation in stable chronic obstructive pulmonary disease patients: a prospective cohort study. J Postgrad Med. 2019;65:101–6.
CAS PubMed PubMed Central Google Scholar
Selzler A-M, Simmonds L, Rodgers WM, Wong EYL, Stickland MK. Pulmonary rehabilitation in chronic obstructive pulmonary disease: predictors of program completion and success. COPD J Chronic Obstr Pulm Dis. 2012;9:538–45.
Li C, Li X, Han H, Cui H, Wang G, Wang Z. Diaphragmatic ultrasonography for predicting ventilator weaning: a meta-analysis. Medicine (Baltimore). 2018;97:e10968.
Rabinovich RA, Ardite E, Troosters T, Carbo N, Alonso J, Gonzalezde Suso JM, Vilaro J, Barbera JA, Polo MF, Argiles JM, et al. Reduced muscle redox capacity after endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1114–8.
Lewinska A, Shahnazaryan K. The use of diaphragm ultrasonography in pulmonary physiotherapy of COPD patients: a literature review. J Clin Med 2020; 9.
Gibson GJ, Whitelaw W, Siafakas N, Supinski GS, Fitting JW, Bellemare F, Loring SH, Troyer AD, Grassino AE. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624.
Summerhill EM, El-Sameed YA, Glidden TJ, McCool FD. Monitoring recovery from diaphragm paralysis with ultrasound. Chest. 2008;133:737–43.
McKenzie DK, Butler JE, Gandevia SC. Respiratory muscle function and activation in chronic obstructive pulmonary disease. J Appl Physiol. 2009;107:621–9.
Laveneziana P, Albuquerque A, Aliverti A, Babb T, Barreiro E, Dres M, Dubé BP, Fauroux B, Gea J, Guenette JA, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J 2019; 53.
Bhatt SP, Luqman-Arafath TK, Gupta AK, Mohan A, Stoltzfus JC, Dey T, Nanda S, Guleria R. Volitional pursed lips breathing in patients with stable chronic obstructive pulmonary disease improves exercise capacity. Chron Respir Dis. 2013;10:5–10.
Corbellini C, Boussuges A, Villafane JH, Zocchi L. Diaphragmatic mobility loss in subjects with moderate to very severe COPD may improve after in-patient pulmonary rehabilitation. Respir Care. 2018;63:1271–80.
Download references
Acknowledgements
Not applicable.
This work was supported by Grants-in-Aid for Scientific Research (21K11325).
Author information
Authors and affiliations.
Department of Rehabilitation Medicine, Kindai University School of Medicine, 377-2 Onohigashi, Osakasayama, Osaka, 5898511, Japan
Masashi Shiraishi, Yuji Higashimoto, Ryuji Sugiya, Hiroki Mizusawa, Yu Takeda, Shuhei Fujita, Tamotsu Kimura & Kanji Fukuda
Department of Respiratory Medicine and Allergology, Kindai University School of Medicine, Osaka, Japan
Masashi Shiraishi, Osamu Nishiyama, Yuji Tohda & Hisako Matsumoto
Inclusive Medical Science Research Institute, Morinomiya University of Medical Sciences, Osaka, Japan
Shintarou Kudo
Division of Biostatistics, Clinical Research Center, Kindai University School of Medicine, Osaka, Japan
Yasutaka Chiba
You can also search for this author in PubMed Google Scholar
Contributions
MS, YH, and YC made substantial contributions to the conception and design of the work. MS, YH, and RS made substantial contributions to the data acquisition. MS and HM made substantial contributions to the analysis. All of the listed authors designed the study and were involved in the interpretation of the data. MS and HM drafted the work. YH, MS, TK, YC, ON, KS, KF, YT, and HM revised the report critically for important intellectual content. All authors approved the final version to be published and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Masashi Shiraishi .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Ethics Committee of Kindai University School of Medicine (31-086). Written informed consent was obtained from all participants.
Consent for publication
If the manuscript is accepted, we approve it for publication in Respiratory Research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Shiraishi, M., Higashimoto, Y., Sugiya, R. et al. Diaphragmatic excursion is correlated with the improvement in exercise tolerance after pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Respir Res 22 , 271 (2021). https://doi.org/10.1186/s12931-021-01870-1
Download citation
Received : 09 July 2021
Accepted : 15 October 2021
Published : 22 October 2021
DOI : https://doi.org/10.1186/s12931-021-01870-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diaphragmatic excursion
- Six-minute walk distance (6MWD)
Respiratory Research
ISSN: 1465-993X
- General enquiries: [email protected]
Comparison of clinical utility between diaphragm excursion and thickening change using ultrasonography to predict extubation success
Affiliations.
- 1 Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 2 Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Gyeongsang National University Hospital, Jinju, Korea.
- PMID: 29050461
- PMCID: PMC5840594
- DOI: 10.3904/kjim.2016.152
Background/aims: Both diaphragmatic excursion and change in muscle thickening are measured using ultrasonography (US) to assess diaphragm function and mechanical ventilation weaning outcomes. However, which parameter can better predict successful extubation remains to be determined. The aim of this study was to compare the clinical utility of these two diaphragmatic parameters to predict extubation success.
Methods: This study included patients subjected to extubation trial in the medical or surgical intensive care unit of a university-affiliated hospital from May 2015 through February 2016. Diaphragm excursion and percent of thickening change (Δtdi%) were measured using US within 24 hours before extubation.
Results: Sixty patients were included, and 78.3% (47/60) of these patients were successfully extubated, whereas 21.7% (13/60) were not. The median degree of excursion was greater in patients with extubation success than in those with extubation failure (1.65 cm vs. 0.8 cm, p < 0.001). Patients with extubation success had a greater Δtdi% than those with extubation failure (42.1% vs. 22.5%, p = 0.03). The areas under the receiver operating curve for excursion and Δtdi% were 0.836 (95% confidence interval [CI], 0.717 to 0.919) and 0.698 (95% CI, 0.566 to 0.810), respectively ( p = 0.017).
Conclusions: Diaphragm excursion seems more accurate than a change in the diaphragm thickness to predict extubation success.
Keywords: Diaphragm; Excursion; Extubation; Thickness; Ultrasonography.
Publication types
- Comparative Study
- Airway Extubation* / statistics & numerical data
- Diaphragm / diagnostic imaging*
- Diaphragm / physiopathology
- Middle Aged
- Prospective Studies
- Retrospective Studies
- Ultrasonography
- Ventilator Weaning / statistics & numerical data

- Students & Professionals
Diaphragmatic Weakness & Paralysis
A weak or paralyzed diaphragm often goes misdiagnosed and left untreated, causing breathing issues that can worsen over time. While there are several medical treatments options, surgery remains the most effective way to treat a paralyzed or weakened diaphragm.
What is the Diaphragm?
The diaphragm is a large muscle that sits below the lungs and heart. The diaphragm is important as it is the primary muscle that facilitates breathing. When the diaphragm contracts, it becomes smaller, causing the lungs to expand in the chest cavity and allowing air to move into the lungs (inhaling). When it relaxes, it enlarges, causing a decrease in lung size thus forcing air out (exhaling).
The diaphragm is controlled by the phrenic nerve, a nerve that is attached to the cervical spine, the area of the spinal cord found in your neck. A paralyzed diaphragm is rarely caused by an injury to the diaphragm itself, but rather by an injury to the phrenic nerve or cervical spine.
What is Diaphragm Weakness or Paralysis?
Patients with a paralyzed diaphragm experience weakness of the diaphragm and have reduced breathing capabilities or are unable to control their voluntary breathing. They also have difficulty maintaining adequate gas exchange, as the lungs are not able to inhale and exhale outside air as efficiently. This is because the phrenic nerve is sending weak signals to the diaphragm to relax or contract, or is unable to send any signal part of or the entire diaphragm.
Diaphragm paralysis can be unilateral or bilateral .
Unilateral paralysis involves one side of the diaphragm. This means that the diaphragm is partially functioning, and the part that is paralyzed will move higher into the chest cavity, taking up space meant for the lungs and interfering with breathing.
Bilateral paralysis occurs when the entire diaphragm is paralyzed. This means that the diaphragm is unable to function in inhalation and exhalation and often requires a machine to assist with breathing.
What Causes Diaphragm Paralysis and Weakness?
Diaphragmatic weakness or paralysis is caused by damage or pressure on the phrenic nerve. There are several known causes that can lead to diaphragm paralysis:
- Birth defects such as congenital central hypoventilation syndrome
- Diseases of the nervous system, such as amyotrophic lateral sclerosis (ALS) or multiple sclerosis
- Injury, such as an upper cervical spinal cord injury that has spared the phrenic nerve
- Phrenic nerve fraying or damaging following cardiothoracic or pulmonary surgery
- Cervical spine arthritis
- Cancer that has spread and compresses the phrenic nerve.
While the cause can be identified in some cases, as many as 40 to 50% of paralyzed diaphragms are idiopathic, meaning the cause is unknown.
What are the symptoms of diaphragmatic weakness and paralysis?
Because the diaphragm plays a central role in breathing, the symptoms are more apparent when both sides of the diaphragm are affected. Symptoms of bilateral diaphragmatic weakness and paralysis include:
- Difficulty breathing, both at rest and when active
- Difficulty sleeping
- Recurrent pneumonia
In unilateral diaphragmatic paralysis, which affects only one side of the diaphragm, dyspnea – or difficulty breathing – on exertion or when lying down is the most typical symptom.
How are diaphragmatic weakness and paralysis diagnosed?
Sometimes diaphragmatic weakness and paralysis is an incidental finding with imaging such as x-rays, MRIs, or ultrasounds. Other times, the diagnosis can be made with blood tests, such as arterial blood gas analysis, that can reveal a lack of needed oxygen in the bloodstream. Pulmonary function tests may also be performed to help confirm diaphragm issues.
What is the treatment for diaphragmatic weakness?
Depending on the severity of injury to the diaphragm, some doctors recommend non-surgical options to treat the breathing issues associated with diaphragm weakness and paralysis. Surgical treatment is an option for more advanced cases or if breathing becomes so impacted daily life is affected.
- Diaphragmatic Pacing : If the phrenic nerve is intact, diaphragmatic pacing is an option. Diaphragmatic pacing is a minimally-invasive surgical option that involves placing a pacemaker to regulate breathing by electrically stimulating the phrenic nerve.
- Diaphragm Plication : Plication is another surgical option for treating a paralyzed diaphragm. Plication involves tying the affected side of the diaphragm to the side that is still functioning normally. This prevents the weakened diaphragm from becoming elevated in the chest cavity and allows the lungs to expand more efficiently and making breathing easier.
Mechanical ventilation with a breathing machine might be required in some more advanced cases.
If you need help for a diaphragm issue, we’re here for you. Call (212) 305-3408 for existing patients, (212) 304-7535 for new patients, or request an appointment online to get started today.
Related Services
- Diaphragm Center See all Related Services
Related Topics
- Diaphragm eventration
- Diaphragmatic hernias
- Diaphragmatic Pacing See all Related Topics
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Diaphragmatic excursion is correlated with the improvement in exercise tolerance after pulmonary rehabilitation in patients with chronic obstructive pulmonary disease
Masashi shiraishi.
1 Department of Rehabilitation Medicine, Kindai University School of Medicine, 377-2 Onohigashi, Osakasayama, Osaka 5898511 Japan
2 Department of Respiratory Medicine and Allergology, Kindai University School of Medicine, Osaka, Japan
Yuji Higashimoto
Ryuji sugiya, hiroki mizusawa, shuhei fujita, osamu nishiyama, shintarou kudo.
3 Inclusive Medical Science Research Institute, Morinomiya University of Medical Sciences, Osaka, Japan
Tamotsu Kimura
Yasutaka chiba.
4 Division of Biostatistics, Clinical Research Center, Kindai University School of Medicine, Osaka, Japan
Kanji Fukuda
Hisako matsumoto, associated data.
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
In patients with chronic obstructive pulmonary disease (COPD), the maximum level of diaphragm excursion (DE max ) is correlated with dynamic lung hyperinflation and exercise tolerance. This study aimed to elucidate the utility of DE max to predict the improvement in exercise tolerance after pulmonary rehabilitation (PR) in patients with COPD.
This was a prospective cohort study. Of the 62 patients with stable COPD who participated in the outpatient PR programme from April 2018 to February 2021, 50 completed the programme. Six-minute walk distance (6MWD) was performed to evaluate exercise tolerance, and ultrasonography was performed to measure DE max . Responders to PR in exercise capacity were defined as patients who demonstrated an increase of > 30 m in 6MWD. The receiver operating characteristic (ROC) curve was used to determine the cut-off point of DE max to predict responses to PR.
Baseline levels of forced expiratory volume in 1 s, 6MWD, maximum inspiratory pressure, DE max and quadriceps muscle strength were significantly higher, and peak dyspnoea of modified Borg (mBorg) scale score was lower in responders (n = 30) than in non-responders (n = 20) to PR (p < 0.01). In multivariate analysis, DE max was significantly correlated with an increase of > 30 m in 6MWD. The area under the ROC curve of DE max to predict responders was 0.915, with a sensitivity and specificity of 83% and 95%, respectively, at a cut-off value of 44.9 mm of DE max .
DE max could adequately predict the improvement in exercise tolerance after PR in patients with COPD.
Chronic obstructive pulmonary disease (COPD) is a progressive disease characterised by minimally reversible airflow limitation [ 1 ]. The main feature of COPD is the inability of patients to cope with their activities of daily life due to shortness of breath. Although the pathophysiological mechanisms involved in the development of dyspnoea and poor exercise tolerance in patients with COPD are complex, dynamic lung hyperinflation (DLH) plays a central role [ 2 ] by increasing ventilatory workload and decreasing the pressure-generating capacity of the inspiratory muscles.
Pulmonary rehabilitation (PR) is a non-pharmacological intervention and has been reported to improve dyspnoea, exercise capacity and quality of life of patients with COPD [ 3 ]. Owing to a body of evidence, PR is now established as the standard of care for patients with COPD [ 4 ]. However, not all patients with COPD benefit from PR to the same extent. Therefore, identifying patients who are likely to achieve maximum benefit from the PR programme is crucial. So far, several studies have shown that severe airflow limitation or poor exercise tolerance at baseline may predict a better response to PR [ 5 , 6 ], but another study has reported inconsistent findings [ 7 ]. Furthermore, one study reported that patients with severe dyspnoea did not respond well to PR and patients with milder dyspnoea responded well [ 8 ].
Considering the role of DLH in the development of dyspnoea and poor exercise tolerance in patients with COPD, objective measures that reflect the degree of DLH may help in identifying good responders to PR. Previously, we reported that there was an association between increased dyspnoea due to DLH on exercise and decreased exercise capacity in patients with COPD and reduced mobility of the diaphragm, which was assessed by the maximum level of diaphragm excursion (DE max ) using ultrasonography [ 9 ]. Other research groups reported the utility of ultrasonographic assessment of diaphragmatic mobility in COPD in understanding its association with 6-min walk distance (6MWD), dyspnoea [ 10 ] and increased mortality [ 11 ].
However, there have been no reports on the association between diaphragmatic mobility and the effect of PR to improve exercise tolerance. The primary aim of this study is to clarify the role of DE max to predict the improvement in exercise tolerance after PR in patients with COPD.
Materials and methods
Study design and subjects.
This was a single-centre, observational, prospective cohort study. The study included 62 patients with clinically stable COPD who visited the Department of Respiratory Medicine and Allergology, Kindai University Hospital, between April 2018 and February 2021. The exclusion criteria included unstable medical conditions that could cause or contribute to breathlessness, such as metabolic, cardiovascular or other respiratory diseases, or any other disorders that could interfere with exercise testing, such as neuromuscular diseases or musculoskeletal problems. This study was approved by the Ethics Committee of Kindai University School of Medicine. Written informed consent was obtained from all participants.
Measurements
All participants underwent ultrasonography (Xario 200, Toshiba, Tokyo, Japan) for the assessment of their DE max . Using the liver as an acoustic window (Fig. 1 A), a convex 3.5 MHz probe was used to measure the excursions of the right hemidiaphragm according to the techniques mentioned in previous studies [ 9 , 12 , 13 ]. The M-mode cursor was rotated and placed on the axis of diaphragmatic displacement on the stored image, and displacement measurements were performed. Measurements were performed during each of the three deep breaths, and DE max was measured (Fig. 1 B). The maximum value obtained for the three deep breaths was used. 6MWD was performed to evaluate walking capacity according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) statement [ 14 – 16 ]. All participants performed the 6MWD test before and after the PR programme, and the magnitude of their perceived breathlessness and their leg fatigue was rated using a 1–10-point Borg scale. Responders to PR in exercise capacity were defined as those who demonstrated more than 30 m increase in 6MWD after the PR programme, which was the definition of minimal clinically important difference (MCID) for 6MWD [ 17 ].

Representative image of the right diaphragm. The probe was positioned below the right costal margin between the midclavicular and anterior axillary lines. A Two-dimensional ultrasonographic image of the right hemidiaphragm (B-mode). Diaphragmatic movements were recorded in M-mode during deep breathing (DE max ) ( B )
Spirometry (CHESTAC-800, Chest, Tokyo, Japan) was performed following the 2005 ATS/ERS recommendations [ 18 ] for measuring forced vital capacity (FVC), forced expiratory volume in 1 s (FEV 1 ) and inspiratory capacity. Respiratory muscle strength was assessed by measuring the maximum inspiratory pressure (PI max ) generated against an occluded airway at residual volume [ 19 ] (SP-370, Fukuda Denshi, Tokyo, Japan). A hand-held dynamometer (μTasF-1, Anima Corp., Tokyo) was used to measure quadriceps muscle strength (QMS). The impact of COPD on health status was assessed using the COPD assessment test (CAT), a patient-completed questionnaire on eight items, namely, cough, phlegm, chest tightness, breathlessness, limited activities, confidence leaving home, sleeplessness and energy. The scores for each of the items range from 0 to 5 points, resulting in a CAT total score ranging from 0 to 40 points [ 20 ], and MCID of CAT is 2 points [ 21 ]. In all patients with COPD, emphysema was evaluated by computed tomography of the chest. A SYNAPSE VINCENT volume analyser (FUJIFILM Medical, Tokyo, Japan) was used to measure the low attenuation area (%LAA).
Rehabilitation programme
The outpatient PR programme was conducted twice a week for 12 weeks (24 sessions), including aerobic exercise training (ergometer and walking exercise) at 60–70% of peak workload for 20–40 min and upper- and lower-limb muscle strength training for 10–20 min.
Sample size
The sample size was estimated using R software. The analysis based on 6MWD data from the PR programme revealed that 40 subjects were required if the expected area under the curve (AUC) below the receiver operating characteristic (ROC) curve was 0.80, the power was 90%, and the significance level was 0.01. Furthermore, we anticipated a dropout from the PR programme. Thus, we set the sample size to 50 participants.
Statistical analysis
Responders and non-responders were compared using t -test, the Wilcoxon rank-sum test or χ 2 test, as appropriate. The paired t -test or the Wilcoxon signed-rank test was used to evaluate the changes in the parameters before and after the PR programme. The Pearson correlation coefficient was used to analyse the relationship between changes in 6MWD and independent variables because changes in 6MWD were normally distributed. Additionally, multivariate logistic regression models were used to assess the ability of variables to predict a response to PR. The ROC curve method was used to assess the ability of DE max to predict a response to PR. All statistical analyses were performed using the JMP software programme (JMP®, Version 14; SAS Institute Inc., Cary, NC, USA).
Out of the 62 patients included in the study, 50 completed the PR programme (Fig. 2 ). Two patients dropped out because of severe exacerbation of COPD, and 10 patients discontinued the PR owing to the coronavirus pandemic. Table Table1 1 presents the baseline characteristics of the participants. After the PR programme, scores for CAT, 6MWD, peak dyspnoea and leg fatigue of the modified Borg (mBorg) scale, and QMS improved significantly (Table (Table2). 2 ). Thirty patients showed an increase of > 30 m in 6MWD after PR (responders: 60%), and 20 patients (40%) were defined as non-responders. Baseline levels of %FEV 1 , 6MWD, PI max , DE max and QMS were significantly higher and those of CAT score and peak dyspnoea of mBorg scale were significantly lower in responders than in non-responders (Table (Table1). 1 ). Changes in 6MWD were significantly correlated with baseline levels of CAT, %FEV 1 , peak dyspnoea of mBorg scale, PI max , DE max (Fig. 3 ) and QMS and marginally correlated with baseline levels of 6MWD (Table (Table3 3 ).

Study flow diagram. COPD chronic obstructive pulmonary disease, PR pulmonary rehabilitation, 6MWD 6-min walk distance
Baseline characteristics of study participants
COPD chronic obstructive pulmonary disease, BMI body mass index, GOLD Global Initiative for Chronic Obstructive Lung Disease, LTOT long-term oxygen therapy, CAT COPD assessment test, FVC forced vital capacity, FEV 1 forced expiratory volume in 1 s, SpO 2 saturation of percutaneous oxygen, LAA low attenuation area, 6MWD 6-min walk distance, mBorg modified Borg, PI max maximum inspiratory pressure, DE max maximum diaphragmatic excursion, QMS quadriceps muscle strength. Values are presented as means ± standard deviations or median (inter-quartile)
Effects of pulmonary rehabilitation (n = 50)
CAT COPD assessment test, 6MWD 6-min walk distance, mBorg modified Borg, QMS quadriceps muscle strength. Values are presented as means ± standard deviations or median (inter-quartile)

Relationship between DE max and the changes in 6MWD after pulmonary rehabilitation. Changes in 6MWD were significantly positively correlated with DE max (r = 0.72; p < 0.001). DE max maximum diaphragmatic excursion, 6MWD 6-min walk distance
Correlations between changes in 6MWD with diaphragm excursion and baseline characteristics
BMI body mass index, CAT COPD assessment test, FVC forced vital capacity, FEV 1 forced expiratory volume in 1 s, 6MWD 6-min walk distance, mBorg modified Borg, PI max maximum inspiratory pressure, DE max maximum diaphragmatic excursion, QMS quadriceps muscle strength
In multivariate analysis, DE max alone significantly contributed to the prediction of responders (Table (Table4, 4 , Model 1). When using PI max instead of DE max because PI max and DE max showed a strong association (r = 0.73), both PI max and %FEV 1 contributed to the prediction (Table (Table4, 4 , Model 2). The area under the ROC curve of DE max to predict the responders was 0.915, with a sensitivity of 83% and a specificity of 95% at a cut-off value of 44.9 mm of DE max (Fig. 4 ). The significance of DE max in the predictability of responders remained even when the analysis was confined to severe patients (%FEV 1 < 50%, n = 23; AUC = 0.88, sensitivity = 70% and specificity = 100% at a cut-off value of 44.9 mm).
Multivariate analysis for responders to pulmonary rehabilitation
FEV 1 forced expiratory volume in 1 s, 6MWD 6-min walk distance, mBorg modified Borg, PI max maximum inspiratory pressure, DE max maximum diaphragmatic excursion, QMS quadriceps muscle strength. † Variables not included in the model

Receiver operating characteristic (ROC) curve for baseline DE max in relation to the response to pulmonary rehabilitation. ROC curve estimates the ability of DE max to predict a clinically important improvement in 6MWD (> 30 m) after pulmonary rehabilitation (AUC = 0.915, sensitivity = 83% and specificity = 95% at a cut-off point of 44.9 mm of DE max ). AUC area under the curve, 6MWD 6-min walk distance, DE max maximum diaphragmatic excursion
This is the first study to demonstrate the utility of DE max to predict the responsiveness of patients with COPD to 12-week PR. In this study, multivariate analysis revealed that greater baseline DE max was the only factor that predicted the responsiveness to PR, independent of baseline %FEV 1 . Additionally, the model using DE max had better prediction performance than that using PI max . The AUC of DE max to predict the 30 m or more improvement in 6MWD after the PR was 0.915, with a sensitivity of 83% and a specificity of 95% at 44.9 mm.
PR is beneficial to patients with chronic respiratory disease, including COPD [ 3 ], and generally improves exercise performance, health-related quality of life and dyspnoea [ 22 ], which was confirmed in this study. Ideally, PR was proven to be effective in all patients, but the response to PR varies considerably between individual patients [ 8 , 23 – 25 ]. Indeed, in this study, the improvement in 6MWD was less than that in MCID in 40% of the patients regardless of the degree of severity of COPD. Therefore, identifying predictors of a response is crucial in ensuring better PR efficacy and personalisation of PR programmes for patients with COPD.
In this study, the baseline values of %FEV 1 , PI max , DE max , QMS and 6MWD were positively associated with Δ6MWD in univariate analysis, suggesting that a better baseline condition was associated with a higher proportion of patients who achieved MCID after PR. These findings are consistent with those of previous studies that showed that patients with higher levels of %FEV 1 or FEV 1 /VC achieved greater improvement in 6MWD after PR [ 7 , 26 , 27 ] and a study in which patients with milder mMRC scores could achieve MCID of 6MWD after PR [ 8 ], but not for those with worst mMRC score, although others studies showed contradictory results [ 5 , 6 , 28 – 30 ] or found no significant baseline characteristics to predict a response to PR [ 31 ]. The discrepancy between the findings cannot be fully explained, but it might be due to the differences in the studied population and strength or length of PR. In this study, the mean %FEV 1 of the participants was 56.0%, which was relatively higher than that of other studies (mean %FEV 1 of 40–50% in most studies) [ 5 , 6 , 28 ], despite similar inclusion criteria throughout the studies, i.e., not limited to severe COPD in most studies. Thus, no ceiling effect with a PR programme that included high-intensity load exercise training for 20–40 min was observed in our population.
In this study, an important finding is that greater DE max at baseline was the only factor that predicted the responders in 6MWD after PR. In addition, the model using DE max had better prediction performance than that using PI max . The high predictability of DE max may be because of its strong association with DLH and dyspnoea during exercise, as reported previously [ 9 ]. DLH is involved in the development of dyspnoea, and both are important factors to determine the improvement in 6MWD in patients with COPD. Therefore, DE max that reflects the degree of DLH and dyspnoea during exercise was superior to other physiological indices to predict responders.
Furthermore, the virtuous cycle observed in our PR programme that included high-intensity load exercise training might be a result of the improvement in ventilation pattern. Improving the ventilation pattern would be easier with greater DE max , as shown in studies of mechanically ventilated patients [ 32 ], which may have reduced dyspnoea during exercise after 12 weeks of PR and improved exercise tolerance. Exercise therapy is a central component of PR, which significantly reduces blood lactate levels during exercise, reduces minute ventilation and improves exercise tolerance [ 33 ]. The high-intensity load exercise training, which is performed at 60–80% of the maximum oxygen uptake, has a higher physiological effect than low exercise load. Patients with greater DE max may be able to perform higher load training, which resulted in effective PR.
Diaphragm ultrasonography has been widely and successfully used to identify diaphragmatic dysfunction by showing its association with 6MWD, dyspnoea [ 10 ], extubation failure in mechanically ventilated patients [ 32 ], and increased mortality [ 11 ]. Recently, Lewinska and Shahnazzaryan proposed its use in pulmonary physiotherapy of patients with COPD [ 34 ]. In most previous studies, diaphragm ultrasonography was used to assess DE max , i.e., the measurement of the excursion of the right hemidiaphragm, as used in this study, and diaphragm thickness that assessed the length and thickness of the zone of apposition of the diaphragm against the rib cage [ 35 , 36 ]. However, it is difficult to measure diaphragm thickness in patients with severe COPD because the length of the zone of apposition is shorter in patients with COPD than that in control subjects [ 37 ], whereas it is easy to measure DE max, which shows high intra- and inter-observer reliability [ 38 ]. Bhatt et al. showed that improvement in 6MWD was associated with that in DE max during forced expiration when the effectiveness of pursed lips breathing was assessed in the PR of patients with COPD [ 39 ]. Corbellini et al. demonstrated greater improvement in DE max during inspiration after PR, which was associated with an increase in the inspiratory capacity [ 40 ]. The normal and cut-off values of DE max during normal respiration, forced respiration, and voluntary sniffing have been described for each gender [ 38 ]. Thus, DE max would be a useful and reliable measure for incorporation into the PR assessment. Furthermore, in clinical settings, this objective measure of DE max has additional advantages as it requires minimum effort in patients and can be applied to the PR programme at home if portable ultrasonography is used. However, the assessment of DE max has a limitation. The procedures pertaining to positioning of patients, breathing patterns, and the selected hemidiaphragm are not standardised at present, which may hamper the routine use of DE max at this moment. Standardisation of these parameters would further facilitate the use of DE max in clinical settings and for research purpose.
There are some limitations to this study. This was a single-centre study involving a relatively small number of participants, and their baseline condition might have been relatively preserved. Nonetheless, 46% of the participants showed FEV 1 < 50%, and the utility of DE max was also observed in these patients with severe airflow limitation. Furthermore, in this study, few patients discontinued the PR programme, except for patients who discontinued during the coronavirus pandemic, which indicates that there was no severe mismatch between the PR programme and the patients’ ability to successfully complete this programme. As another limitation, we did not evaluate any malnutrition factors, which could be an important determinant of diaphragmatic mobility. Nonetheless, DE max was a stronger predictor of the effectiveness of PR than other parameters, including QMS or lung function using multivariate analysis. Further studies with a large number of patients are required, and the utility of DE max should be examined in patients with the most severe form of COPD with a low-intensity load exercise programme.
In conclusion, DE max , which is a reliable and easy to perform measurement, could adequately predict the improvement in exercise tolerance after PR in patients with COPD. Assessment of DE max could aid in making medical decisions associated with therapeutic strategies.
Acknowledgements
Not applicable.
Abbreviations
Authors’ contributions.
MS, YH, and YC made substantial contributions to the conception and design of the work. MS, YH, and RS made substantial contributions to the data acquisition. MS and HM made substantial contributions to the analysis. All of the listed authors designed the study and were involved in the interpretation of the data. MS and HM drafted the work. YH, MS, TK, YC, ON, KS, KF, YT, and HM revised the report critically for important intellectual content. All authors approved the final version to be published and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
This work was supported by Grants-in-Aid for Scientific Research (21K11325).
Availability of data and materials
Declarations.
This study was approved by the Ethics Committee of Kindai University School of Medicine (31-086). Written informed consent was obtained from all participants.
If the manuscript is accepted, we approve it for publication in Respiratory Research.
The authors declare no competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
The mean diaphragmatic excursions of the two hemidiaphragms have been determined for men and women (Table (Table1). 1). Furthermore, in 1995, Houston et al have reported that in healthy volunteers, the right-to-left ratio of hemidiaphragmatic excursion during deep inspiration was in the range of 0.5-1.6. Consequently, this ratio has been ...
Check us out on Facebook for DAILY FREE REVIEW QUESTIONS and updates! (https://www.facebook.com/medschoolmadeeasy) Check out our website for TONS OF FREE REV...
Diaphragmatic excursion values presented in this study can be used as reference values to detect diaphragmatic dysfunction in clinical practice. There is a significant statistical difference between right and left hemidiaphragmatic movement during all types of breathing (quiet, deep and sniffing). ...
Furthermore, diaphragmatic excursion is an index for respiratory muscle fatigue during the SBT. Some authors had reported a lower accuracy for diaphragmatic excursion compared to most of the available data and suggested that this lower accuracy is due to the heterogeneity of the patients included in the meta-analyses , . Therefore, separate ...
The fluoroscopic sniff test, also known as diaphragm fluoroscopy, is a quick and easy real time fluoroscopic assessment of diaphragmatic motor function (excursion).It is used most often to confirm absence of muscular contraction of the diaphragm during inspiration in patients with phrenic nerve palsy or breathing difficulties following stroke.Chest radiograph demonstrating a newly elevated ...
Diaphragmatic excursion: Can be evaluated via percussion. Is 4-6 centimeters between full inspiration and full expiration. May be abnormal with hyperinflation, atelectasis, the presence of a pleural effusion, diaphragmatic paralysis, or at times with intra-abdominal pathology.
The diaphragm is the dome-shaped structure that separates the thoracic and abdominal cavities. ... Decreased diaphragmatic excursion may be detected by percussion of the lower rib cage at end ...
Measure the distance between the marks to determine diaphragmatic excursion, normally 5 to 6 cm in adults. Repeat these steps on your patient's other side and compare. Because the diaphragm is usually higher on the right because of displacement by the liver, the measurement may be greater on the left. 4.
Diaphragmatic excursion is a quantitative measure of expiratory effort as validated by both lung and tracheal volumes in asthma patients, and may be more accurate than qualitative assessment based on tracheal morphology.
The use of ultrasound to visualize the diaphragm is well established. Over the last 15 years, certain indices of diaphragm function, namely diaphragm thickness, thickening fraction and excursion have been established for mechanically ventilated patients to track changes in diaphragm size and function over time, to assess and diagnose diaphragmatic dysfunction, and to evaluate if these indices ...
Diaphragm thickening fraction, which is also measured with ultrasound, is another interesting technique as several studies demonstrated its reliability to predict extubation success [].It might even be superior to diaphragm excursion in this indication: two studies have demonstrated a significant correlation between the diaphragmatic tidal thickening fraction and the diaphragmatic pressure ...
Diaphragmatic ultrasound can also be used in order to predict the weaning outcome. During spontaneous breathing trials, diaphragmatic excursion cutoff values of < 14 mm 53 , 54 and < 11 mm 13 have both been found to be predictive of weaning failure, as have TF values of < 20%, 43 < 30%, 46 and < 36%. 11.
Diaphragmatic excursion (DE) was calculated as the difference between axial slices through the lungs on inspiration and expiration, using the first slice at which the lung apex was evident as the cranial bound, and the first slice at which the diaphragm appeared as the caudal bound. Slices were then multiplied by slice thickness, to yield the ...
Diaphragmatic thickness (Tdi) is measured by placing a high-frequency linear probe at the level of the zone of apposition, while diaphragmatic excursion is measured by placing a curvilinear probe in the subcostal region and recording diaphragmatic movements in the M-mode.
In patients with chronic obstructive pulmonary disease (COPD), the maximum level of diaphragm excursion (DEmax) is correlated with dynamic lung hyperinflation and exercise tolerance. This study aimed to elucidate the utility of DEmax to predict the improvement in exercise tolerance after pulmonary rehabilitation (PR) in patients with COPD. This was a prospective cohort study.
The diaphragmatic excursion was higher in males than females. There was a significant difference in diaphragmatic excursion among age groups. Age, sex and BMI significantly affected the diaphragmatic motion. Conclusions: Diaphragmatic excursion values presented in this study can be used as reference values to detect diaphragmatic dysfunction in ...
Diaphragmatic excursion measured with ultrasound is increasin-gly useful in the thoracoabdominal assessment of patients. Perhaps the most widely used is in the critically ill patient, to define muscle deconditioning, which is crucial in predicting successful extubation in
Diaphragmatic excursions are sensitive to changes in respiratory patterns , are related to the volume-generating capacity of the diaphragm (measured by VC) following abdominal surgery , and have been used to identify diaphragmatic weakness in the setting of the acute exacerbation of COPD . In this ...
Background/aims: Both diaphragmatic excursion and change in muscle thickening are measured using ultrasonography (US) to assess diaphragm function and mechanical ventilation weaning outcomes. However, which parameter can better predict successful extubation remains to be determined. The aim of this study was to compare the clinical utility of these two diaphragmatic parameters to predict ...
Diaphragmatic weakness or paralysis is caused by damage or pressure on the phrenic nerve. There are several known causes that can lead to diaphragm paralysis: Cancer that has spread and compresses the phrenic nerve. While the cause can be identified in some cases, as many as 40 to 50% of paralyzed diaphragms are idiopathic, meaning the cause is ...
The study establishes that COPD affects diaphragmatic excursion and lung function. We found that diaphragmatic excursion was reduced in COPD than controls. Decreased diaphragmatic excursion shows that contractile ability of diaphragm is reduced in COPD. The reason of reduced contractility lies in the pathophysiology of the disease.
2 Abstract Purpose: Lung tissue and lung excursion segmentation in thoracic dynamic magnetic resonance 45 imaging (dMRI) is a critical step for quantitative analysis of thoracic structure and function in patients with respiratory disorders such as Thoracic Insufficiency Syndrome (TIS). However, the complex variability of intensity and shape of anatomical structures and the low contrast between
In most previous studies, diaphragm ultrasonography was used to assess DE max, i.e., the measurement of the excursion of the right hemidiaphragm, as used in this study, and diaphragm thickness that assessed the length and thickness of the zone of apposition of the diaphragm against the rib cage [35, 36].